Lichen Planus (LP) is a common chronic inflammatory disease affecting skin, mucous membranes and appendages with an unclear aetiopathogenesis. It affects about 2% of the population with a female predominance [1,2].
Although the aetiology and pathogenesis are not fully understood, it is believed that LP represents a T- cell mediated inflammatory disorder. Inflammation produces disturbances in lipid metabolism such as increased serum triglycerides or decreased High Density Lipoprotein (HDL). These lipid disturbances linked to chronic inflammation participate in the increased risk of cardiovascular disease associated with dyslipidemia. This association has been already established in psoriasis [3]. Epidermal cells in LP have shown abnormalities in enzymatic activity, as well as defective carbohydrate expression. An increased prevalence of diabetes and carbohydrate intolerance has been observed in patients with LP, suggesting its possible role in the pathogenesis.
Metabolic Syndrome (Met S) is a constellation of cardiovascular risk factors including obesity, hypertension, dyslipidemia and insulin resistance. It has been seen to be associated with chronic inflammation [4,5]. Several cytokines (TNF-α, IL-2, IL-6) have been implicated for increased lipid levels in body. Also, TNF-α and IFN-γ have been shown to be present in higher concentrations in the lesions of LP. Among the different components of Met S, dyslipidemia has been found to be significantly associated with LP [6,7].
In developing countries like India, data on lipid parameters in patients suffering from LP are scarce. To the best of our knowledge, few studies have investigated the association between LP and abnormality in lipid and glucose levels and no study has been done so far in the eastern part of India.
To study the association of metabolic derangements in LP.
Materials and Methods
A prospective case control study was undertaken in patients of LP as cases and age and sex matched patients with other non-inflammatory diseases as controls. It was conducted for a period of one year (February 2015 to January 2016) on patients attending the Out-Patient Department (OPD) of dermatology in a tertiary care teaching hospital in Bhubaneswar, Odisha. Diagnosis of LP was based on clinical examination findings of itchy red to violet, flat topped, polygonal, papules and plaques healing with post-inflammatory hyperpigmentation [8]. Other variants of LP like hypertrophic, annular, follicular, actinic, palmoplantar and LP pigmentosus were also included. Diagnosis in doubtful cases were confirmed by the typical histopathological findings of compact hyperkeratosis, acanthosis, focal hypergranulosis, flattened or effaced rete ridges (saw tooth appearance), presence of colloid bodies at the basal layer and band like infiltrate of lymphocytes and histiocytes at the dermo-epidermal junction [8]. The purpose and methodology of the study was explained to each subject individually and their consent for participation was obtained. Data was collected in a predesigned performa. Ethical clearance was taken from the institutional ethical committee.
The following inclusion and exclusion criteria were used to recruit the patients to case and control groups:
Cases - LP (both skin and/or mucosa) patients of both genders between 20-60 years of age were included. Patients having metabolic derangements in the form of obesity, diabetes mellitus, dyslipidemia, hypertension, patients with history of cardiovascular disease in family, patients having lichenoid drug eruption and those who were under systemic treatment for LP were excluded.
Controls – Age and sex matched patients with other non-inflammatory skin diseases attending the dermatology OPD were included as controls. Patients having metabolic derangements as described above, patients having history of cardiovascular disease in family and those who were under systemic therapy for any other disease were excluded.
Clinical Parameters and Biochemical Assays
The following investigations were undertaken for the evaluation of the case study:
1. Weight & height of subjects were measured, and their BMI {Body Mass Index (kg/m2)} was calculated.
2. Serum total cholesterol, triglycerides, High-Density Lipoprotein Cholesterol (HDL-C), Low-Density Lipoprotein Cholesterol (LDL-C), total cholesterol, LDL-C/HDL-C and Total cholesterol/HDL-C were measured.
3. Blood glucose levels were studied in samples drawn after a 12-hour fasting period.
Statistical Analysis
The results were analysed using standard statistical tests. Results were compared between groups by paired t-tests for independent variables and p-values were calculated. Correlation analysis was done by linear regression analysis between variables.
Two-sample student’s t-test was used to compare mean values of quantitative variables as the 2 samples were obtained independently. Correlations among variables were studied by using the Pearson coefficient and exponential regression technique. Binary logistic regression models (Wald method), obtaining estimates adjusted Odds Ratios (OR) and their 95% confidence intervals were used to measure the association between LP and lipid levels in a multivariate analysis. The p-value 0.05 was considered significant in all analyses.
Results
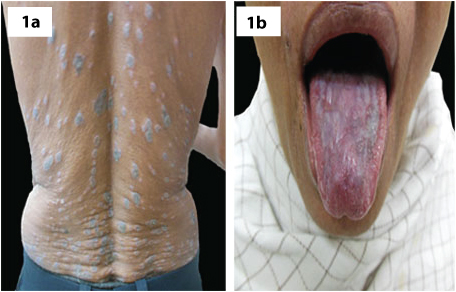
A total of 80 patients were recruited in the study, 40 cases with LP [Table/Fig-1a,b] and 40 controls. Mean age, height, weight and BMI of the study patients are summarized in [Table/Fig-2]. The mean age for cases was 40.5 (SD 9.3) years and controls was 40.3 (SD 5.5) years with p-value of 0.9. The mean height, weight and BMI for cases were 160.1 (SD 11.9) cm, 62.4 (SD 9.4) kg, 24.3 (SD 2.1) and for controls were 158.4 (SD 13.5) cm, 60.3 (SD 11.0) kg and 23.9 (SD 2.1) respectively with p-value of 0.55, 0.36 and 0.39 respectively.
(a) Trunk showing violaceous, flat topped papules and plaques with koebnerisation. (b) Lichen planus involving oral mucosa.
Epidemiological profile comparison of the study patients (Mean±SD).
| No. of Patients | (n=40) | (n=40) | p–value |
|---|
| Case | Control |
|---|
| Age (Year) | 40.5±9.3 | 40.3±5.5 | 0.9 |
| Height (cm) | 160.1±11.9 | 158.4±13.5 | 0.55 |
| Weight (Kg) | 62.4±9.4 | 60.3±11.0 | 0.36 |
| BMI (Kg/cm2) | 24.3±2.1 | 23.9±2.1 | 0.39 |
The total cholesterol, triglycerides, HDL-C, LDL-C, LDL-C/HDL-C, total cholesterol/HDL-C and fasting blood sugar values for cases and controls are listed in [Table/Fig-3]. Patients with lichen planus showed higher significant mean values than controls for all the parameters. The p-values were significant for all the parameters.
Blood parameters comparison of the study patients (Mean±SD).
| No. of Patients | (n=40) | (n=40) | p–value |
|---|
| Case | Control |
|---|
| Cholesterol (mg/dl) | 200.3±17.8 | 177.4±25.3 | <0.0001 |
| Triglyceride (mg/dl) | 151.6±24.4 | 108.1±32.0 | <0.0001 |
| HDL-C (mg/dl) | 42.4±6.1 | 57.1±8.8 | <0.0001 |
| LDL-C (mg/dl) | 117.4±13.3 | 100.1±13.3 | <0.0001 |
| LDL-C/HDL-C | 2.8±0.5 | 1.8±0.3 | <0.0001 |
| Total Cholesterol/HDL-C | 4.8±0.5 | 3.2±0.9 | <0.0001 |
| Fasting Blood Sugar (mg/dl) | 96.0±6.3 | 89.4±5.9 | <0.0001 |
Discussion
The aetiology of LP is currently considered multifactorial with an emphasis on autoimmune process. It is characterized by the upregulation of inflammatory CXCR3 ligands associated with the recruitment of effector cytotoxic T-cells and plasmacytoid dendritic cells [8]. Various cytokines as mentioned above including interleukin-2, IL-4, IL-6, IL-10, TNF-α, IFN-α, IFN-γ and TGF-β1 are involved in LP [9,10]. Recent study [11] has proposed a pro-inflammatory role of NK lymphocytes in LP. The presence of increased numbers of CD56 highCD16 - NK cells in the lesions of LP, which secrete IFN-γ, TNF-α and sometimes IL-22, IL-17 and IL-4 contributes to the pathogenesis.
LP is sometimes associated with oxidative stress and certain medications [12]. A delayed hypersensitivity reaction in which the release of cytokines by activated T-cells attracts inflammatory cells and leads to the destruction of keratinocytes, resulting in generation of reactive oxygen species. During this lymphocytotoxic process the keratinocytes release more cytokines that are epidermotrophic. All these events play a role in the pathogenesis of LP [9,10,13,14]. The underlying chronic pro-inflammatory situation possibly can explain the link between LP, dyslipidemia and other metabolic derangements.
The mean age of our study patients with LP was 40.5 (SD 9.3) years which is comparable to results found in other studies reporting the mean age as 47.4 (SD 9.0) years [7] and 52.8 (SD 15.3) years [6].
Comparing the height and weight of our cases with the study by Arias-Santiago et al., we found mean height as 160.1 (SD 11.9) cm and mean weight as 62.4 (SD 9.4) kg as compared to 167.4 (SD 9.4) cm and 73.9 (SD 12.7) kg respectively in the study [7].
The mean BMI for our patients was found to be 24.3 (SD 2.1) kg/m2 as compared to 26.4 (SD 4.5) kg/m2 found by Arias-Santiago et al., [7].
As described in [Table/Fig-3] the mean values for total cholesterol, triglyceride, HDL-C, LDL-C, LDL-C/HDL-C and total cholesterol/HDL-C for our cases were comparable with that of Arias-Santiago et al., who reported the mean values for lipid parameters in their study as 200.1 (SD 35.6) mg/dl, 149.5 (SD 86.6) mg/dl, 54.5 (SD 12.9) mg/dl, 120.1 (SD 30.0) mg/dl, 2.2 (SD 1.0) and 3.7 (SD 1.2) respectively [7].
The mean fasting blood sugar values of our study patients was 96.0 (SD 6.3) mg/dl comparable to 97.85 (SD 25.45) mg/dl found by Seyhan M et al., and 91.4 (SD 36.1) mg/dl found by Arias-Santiago et al., [1,7].
Dreiher J et al., found that LP was associated with dyslipidemia in a large series of patients [6], however no data were presented about lipid values, glucose levels, abdominal obesity or blood pressure in patients or controls. Also, they did not differentiate between LP and lichenoid drug reactions which are related to several drugs including HMG-CoA reductase inhibitors as well as antihypertensives. Another recent study from Spain [7] found a positive association of LP with dyslipidemia taking into consideration of all the important parameters associated with dyslipidemia.
Krishnamoorthy B et al., studied the lipid profile and Met S status in patients with Oral LP (OLP) and Oral Lichenoid Reaction (OLR) as compared to healthy individuals [15], and found an increased level of serum cholesterol and LDL-C in patients with OLP and OLR. Also, they found high levels of serum triglyceride and VLDL-C and low levels of HDL-C in patients with OLP as compared to patients with OLR. In a recent meta-analysis by Lai YC et al., seven studies with 5242 subjects were analysed. It was found that LP was strongly associated with dyslipidemia and high triglyceride levels [16].
Present study found higher lipid levels in patients with LP. All the lipid parameters were different in men and women with LP. After controlling for sex, age, height, weight and BMI, patients with LP presented higher risk for dyslipidemia. Increased LDL-C/HDL-C ratio has been considered a sensitive predictor of cardiovascular risk and recently, total cholesterol/HDL-C ratio has also been found as a better predictor for cardiovascular risk in a large study [17]. In our study we found higher values of both ratios in the cases as compared to controls.
Abnormal glucose tolerance test in association with LP has been described in earlier studies with 62% [18] and 42% [19] of the subjects showing impaired tolerance. Grinspan in 1966 first reported the association of erosive OLP with diabetes mellitus and arterial hypertension (Grinspan’s syndrome) [20]. Out of 62 patients suffering from OLP, 27.4% were associated with type 2 diabetes mellitus and 17.7% had impaired fasting glucose levels in a study by Romero et al., [21]. Although the difference in fasting glucose levels in our study was statistically significant with a higher value in the group of patients with LP, levels in both the groups were well within normal limits. It is very difficult to conclude from the current study, whether carbohydrate metabolism changes like hyperglycaemia is also seen in LP as a part of its association with Met S.
Limitation
Main limitations of the present study was the inclusion of the less number of patients in the study. Though the sample size for statistically significant analysis of the data would be more than what we have recruited, being a time bound hospital based study we could recruit only 40 patients in each group.
Conclusion
There is a need to undertake larger studies with recruitment of more number of patients, along with additional data so as to establish the association of Met S in LP as already established in other inflammatory skin diseases like psoriasis. Also we emphasize on routine blood investigations in LP patients for early detection and management of dyslipidemia and hyperglycaemia.