The diagnosis of Diffuse Parenchymal Lung Disease (DPLD) requires a multidisciplinary approach with reconciliation of clinical, radiological and histopathological information. Clinical evaluation alone is inadequate in making an aetiological diagnosis of DPLD except in certain specific conditions like connective tissue diseases with typical manifestations. Pattern recognition from a High Resolution Computed Tomography (HRCT) scan of thorax is helpful in making a diagnosis in diseases like Sarcoidosis, Usual Interstitial Pneumonia (UIP), Sub-acute Hypersensitivity Pneumonitis, Lymphangioleiomyomatosis and Cryptogenic Organizing Pneumonia (COP) [1]. In clinical practice, the patient often presents at a late stage or while on corticosteroid treatment, thus masking the typical clinico-radiological clues expected. A representative lung biopsy with careful examination of tissue becomes necessary in such situations. Attempting an invasive procedure like an open surgical lung biopsy in an already compromised respiratory system is a high risk procedure for the surgeon. So, the advantages need to be balanced with the risks due to the procedure. The present retrospective study was undertaken to study the utility and diagnostic yield of Trans-Bronchial Lung Biopsy (TBLB) in DPLD with clinicoradiological discordance.
Materials and Methods
This was a retrospective observational study of TBLB conducted between 1st Jan 2012 and 31st Dec 2014 at the Institute of Chest Diseases, Government Medical College, Kozhikode, Kerala, India.
Inclusion Criteria: All cases of DPLD in whom a definite diagnosis was not possible by a multidisciplinary team with clinical and radiological assessment.
Exclusion Criteria: Severely hypoxemic, medically unstable, bed ridden patients and those who did not consent for biopsy.
The study was approved by the Institutional Ethics Committee, Government Medical College, Kozhikode and an informed written consent was obtained from all the participants before the procedure as part of the institute’s protocol. Confidentiality of all study subjects was maintained.
Trans-Bronchial Lung Biopsy (TBLB) Procedure: All patients underwent a blind TBLB (without fluoroscopic guidance) using a fibre optic video bronchoscope, (Pentax EB-1575K) under topical anaesthesia by an experienced interventional pulmonologist. Most of the TBLB procedures were done on an outpatient basis under mild sedation. Of the total, 30 patients were given Midazolam 1mg and 36 patients received 2mg Midazolam depending on the level of conscious sedation needed. TBLB samples were taken from appropriate lung segments which matched areas of ground glass opacities, consolidation, nodules and reticular shadows on the HRCT lung images. The biopsy was taken using standard biopsy forceps (Pentax-KW2411R). Four to six samples from a single lung were taken per patient. We did not make an imprint smear of the specimens to prevent crush artefact and directly transferred it into formalin fixative.
A check chest radiograph was done post-procedure only if the patient complained of pain and there was a discrepancy in breath sounds. In addition all patients were observed for four hours and instructed to report immediately if there was any aggravation of symptoms. The TBLB specimens were examined and reported by two experienced pathologists after reaching a consensus. All specimens were accompanied by a clinical summary and a probable clinicoradiological diagnosis from our side and the final report was discussed with the lung radiologist and pathologists. For patients in whom the TBLB was inconclusive, an open lung biopsy was performed.
Results
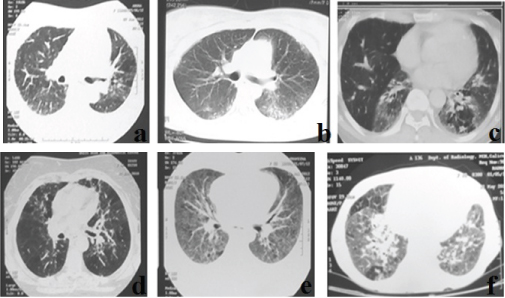
After clinicoradiological assessment of 169 DPLD patients, we had 66 patients in whom the diagnosis was inconclusive. These included 42 female (64%) and 24 male (36%) patients. Of the total 33 patients were above 60 years (50%), 27 patients were in age group 30–59 years (41%) and 6 patients were below the age 30 (9%). The major patterns in HRCT lung sections included ground glassing/consolidation in 45 (68%), reticular shadows in 20 (30%), and septal thickening in 25 (37.8%). Some images also showed nodules in 10 (15%), cysts in 2 (3%), mosaic attenuation in 9 (13.6%) and honey combing in around 22% (predominant upper zonal). If the honey combing fit the criteria for a definite UIP, they were excluded [2]. Few of the representative HRCT lung images are shown in [Table/Fig-1].
Few of the representative HRCT lung sections.
a-f-: Images a and d shows areas of patchy ground glassing and mosaic attenuation; b shows peripheral subpleural patchy ground glassing and consolidation; c shows ground glassing with contracted lower lobes; e shows bilateral diffuse ground glass opacities; f shows bilateral ground glassing with peribronchovascular consolidation in the right lower lobe and patchy areas of mosaic attenuation.
A definite histopathological diagnosis from TBLB specimens were obtained in 39 (59%) out of the 66 patients. In the remaining 27 patients in whom histopathology were inconclusive, 12 patients with reasonably good performance status were taken for open lung biopsy, after getting a high risk informed consent. We could not arrive at a proper diagnosis in 15 patients who did not consent for open lung biopsy. The clinicoradiological diagnosis along with the corresponding histopathological appearance, obtained in the 51 cases are shown in [Table/Fig-2,3]. Pneumocystis jirovecii pneumonia, Aspergillus fungal infection and a case of invasive adenocarcinoma were unsuspected diagnoses revealed in the TBLB specimens [Table/Fig-4,5].
Clinicoradiological and histopathology diagnosis in patients who had TBLB.
| No of cases | Provisional Diagnosis | HRCT Diagnosis Grouping | Representative Histopathological Results | Final Diagnosis |
|---|
| 11 | NSIP | Mid-lower zone consolidation/ground glassing, traction bronchiectasis, upper lobe honeycombing | Chronic inflammation with fibrosis in a homogenous pattern. No granulomas. Scattered areas of honey combing | NSIP |
| 9 | ILD? HP | Peribronchovascular thickening, fibrosis and ground glassing with areas of consolidation. Scattered mosaic attenuation? HP. Associated cysts seen in two cases | Non-caseating granulomas seen with lymphocytic infiltrates. Correlated with BAL lymphocytosis also | Hypersensitivity pneumonitis |
| 6 | ILD-IIP/HP/Sarcoid | Peribronchovascular thickening with few peribronchovascular and centrilobular nodules. Few Mediastinal nodes? Sarcoid/HP/COP | Uniform sized, non-caseating granulomas consistent with sarcoidosis | Sarcoidosis |
| 5 | OP/NSIP | Bilateral peribronchovascular GGO, traction bronchiectasis. No architectural distortion. | Chronic inflammation with granulation tissue | OP |
| 4 | COP/PAP/? Infection | Bilateral GGO/consolidation, few centrilobular nodules. | Atypical cells with papillary pattern. | Adeno Carcinoma |
| 1 | COP/Infection | Bilateral GGO/consolidation | Fungal hyphae demonstrated with chronic inflammatory infiltrate | Aspergillosis |
| 1 | Post renal transplant ?Infection | Bilateral extensive consolidation | Diffuse alveolar exudates. Giemsa stain positive organism in BAL. | Consistent with Pneumocystis jirovecii infection |
| 1 | ILD with oculocutaneous albinism | Consolidation with traction bronchiectasis? Fibrosis with inter and intralobular septal thickening. Upper zone honeycombing? C/c HP/UIP | Fibroblastic foci with chronic inflammation | UIP |
| 1 | ILD with cutaneous syndrome | Consolidation and fibrosis? UIP/Fibrotic NSIP | Chronic inflammation and honey combing | UIP associated with Dyskeratosis congenita |
(ILD-Interstitial Lung Disease, IIP-Idiopathic Interstitial Pneumonia, NSIP-Non Specific Interstitial pneumonia, UIP-Usual interstitial Pneumonia, HP- Hypersensitivity Pneumonitis, COP-Cryptogenic Organising Pneumonia, PAP-Pulmonary Alveolar Proteinosis, GGO-Ground Glass Opacity, C/c-Chronic).
Clinicoradiological and histopathology of patients who had in addition an open lung biopsy.
| Numbers(n=12) | ProvisionalDiagnosis | HRCT Diagnosis | Histopathology | Final Multi-disciplinaryDiagnosis |
|---|
| 5 | IIP | NSIP with progression/Chronic HP | Fibroblastic foci with honeycombing. | UIP |
| 3 | IIP | Bronchiolocentric OP pattern | Chronic inflammation and granulation tissue with areas of fibrosis | COP |
| 2 | IIP | UIP with asymmetrical ground glassing | Fibrosis and honeycombing with normal lung tissue | Acute exacerbation of IPF |
| 2 | IIP/Drug induced | Bilateral consolidation and traction bronchiectasis. Subtle honeycombing | Fibrosis and lymphoplasmacytic infiltration | ILD-Bleomycin induced |
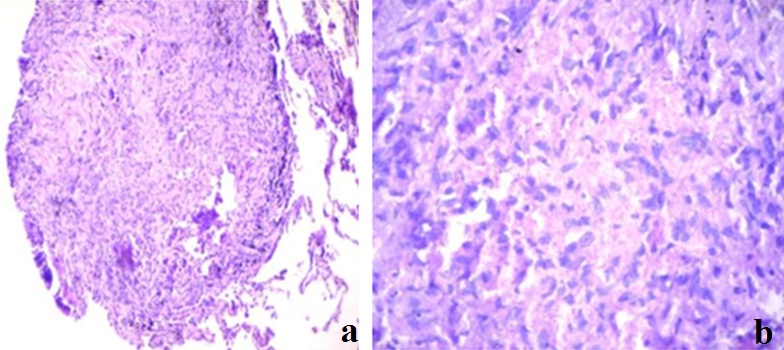
Histopathology of lung section showing invasive adenocarcinoma.
a) Showing transbronchial lung biopsy specimen at magnification 10X10; b) Showing same specimen at 10X100 magnification (Oil immersion H&E stain). Image shows atypical areas with high nuclear cytplasmic ratio.
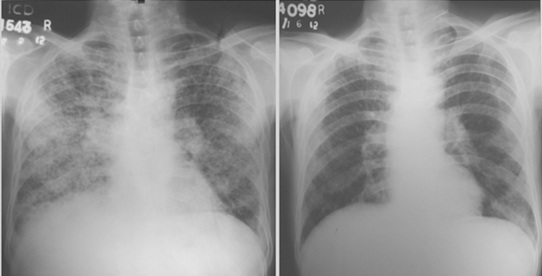
Chest X ray of the patient with fungal infection before and after treatment.
Discussion
The first bronchoscopic lung biopsy was performed by Dr. Anderson in 1963 using a rigid bronchoscope [3]. A decade later, Levin et al., published their experience with a flexible bronchoscopic transbronchial biopsy [4]. TBLB, a relatively safe tool is routinely used by pulmonologists for the diagnosis of DPLD. We did the procedure for the diagnosis of pulmonary infections, unclassifiable Interstitial Lung Diseases (ILD) and neoplasms. The main utility of the TBLB rests in the possibility of making a specific diagnosis in a patient with DPLD and avoiding a surgical lung biopsy. While monomorphous histological patterns like organising pneumonia or alveolar proteinosis are easily identifiable in small lung fragments obtained by TBLB, it may not be sufficient in histologically heterogenous diseases like UIP [5]. Nevertheless, the TBLB forceps makes it possible to obtain lung tissue through the bronchial routes and the specimens obtained usually represent the centrilobular regions. So, it is especially useful in disorders that are centred around terminal/respiratory bronchioles and lymphatics, e.g., Respiratory Bronchiolitis Interstitial Lung Disease (RBILD), Sarcoidosis, Hypersensitivity Pneumonitis (HP) and Lymphangitis carcinomatosis. The diagnostic yield for sarcoidosis ranges from 50% to 85% in stage 2 disease [6]. Our study shows a TBLB yield of around 59% and if combined with the open lung biopsy report in 12 indeterminate cases, a definite diagnosis was possible in about 77% of the cases (51/66).
Berbescu et al., retrospectively examined transbronchial biopsies (TBLB) of 22 patients with known UIP (21 cases diagnosed by surgical biopsy, one case diagnosed by clinical and radiologic features). The diagnosis of UIP was possible in seven and consistent with UIP in two subjects with TBLB. As a rationale for the use of TBLB, the authors reported high mortality within 30 days following surgical lung biopsy for UIP [7]. However, Churg and Schwarz have described that “collective wisdom be followed and that TBLB should not be used to diagnose IPF/UIP” because the morphologic findings of interstitial inflammation and fibrosis in TBLB appeared to have little relevance to the subsequent clinical course [8].
In the present study we have therefore included cases for TBLB only if a definite diagnosis was not possible by clinical and radiological grounds. Ensminger and Prakash, evaluated whether the results of TBLB were clinically useful in the management of patients with diffuse pulmonary disorders. TBLB was considered useful if: (a) it resulted in a specific clinical diagnosis, (b) a specific management decision was made based on the biopsy result, or (c) certain pathologic processes were excluded on the basis of the biopsy result and other clinical information. The results indicated that TBLB was a clinically useful test in approximately 75% of procedures, certainly not a negligible percentage [9].
A retrospective study conducted on ILD diagnosed by TBLB or open lung biopsy showed that TBLB had a diagnostic yield of 80.7% with a very high prevalence of UIP [10]. In our present study we excluded UIP based on the definite radiological diagnostic criteria for UIP [2]. A 3 year prospective study conducted on 49 ILD patients from North India after excluding IPF showed overall diagnostic yield of TBLB to be around 85.7%. Non-specific interstitial pneumonitis, tuberculosis and sarcoidosis were the most common histology patterns found in their study (22.4%, 18.4% and 16.3%, respectively). Inexperience of pathologists in making an accurate diagnosis is stressed in the above study [11].
The sensitivity of TBLB also varies according to the number of biopsy specimens taken. British Thoracic Society (BTS) guidelines recommend 4–6 samples from one lung in diffuse lung disease [12]. We followed the BTS guidelines and got consistent reports with 4-6 tissue samples. Since the tissue samples obtained by means of TBLB forceps are small (approximately 3mm in size), adequate number of samples and mapping areas of biopsy interest prior to bronchoscopy will yield results. Importantly, the areas of fibrosis and honeycombing are not going to yield much to the final result.
Recently the cryoprobe –TBLB, using the flexible bronchoscope is gaining popularity in the diagnosis of DPLD. The diagnostic yield is as high as 80% even in complex cases as larger tissue samples (diameter 4mm-10mm) are obtained without crush artifacts. In a study by Casoni GL et al., out of 69 patients, adequate cryobiopsy specimens were obtained in 68 cases. The median size of cryobiopsies was 43.11mm2 (range, 11.94-76.25mm2). The diagnostic yield among adequate samples was 76% (52 out of 68 cases) including 36 of 47 with UIP (77%) and nine nonspecific interstitial pneumonia (6 fibrosing and 3 cellular) [13]. In a randomised control trial by Pajares V et al., the diagnostic yield was higher for the cryoprobe group (74.4%) than in the conventional-forceps group (34.1%) [14]. Cryo-TBLB may replace surgical lung biopsy in DPLD especially in elderly, those with co-morbidities and high risk patients. Pneumothorax and bleeding are the common adverse events encountered.
TBLB also finds a place in the diagnosis of pulmonary infections in immunocompromised patients. Though a Broncho-Alveolar Lavage (BAL) in isolation can be used for diagnosing infections, a TBLB increases the diagnostic yield and rules out non infectious causes. TBLB is especially useful in solid organ transplant patients who are predisposed for opportunistic infections like Pneumocystis jiroveci pneumonia [15,16]. The post renal transplant patient included in our study, who was on Mycofenolate mofetil, Tacrolimus and Prednisolone significantly improved on Cotrimoxazole treatment. We also had another patient with bilateral reticulonodular infiltrates mimicking DPLD. This patient, in addition had history, clinical findings and pulmonary function testing which corroborated with the diagnosis of a DPLD. Though his BAL study was negative for bacteria and fungi, subsequent to the report of fungal infection on TBLB, he was started on oral Itraconazole and showed dramatic improvement as evident on the follow-up chest skiagram [Table/Fig-5].
Complications: Open lung biopsy is associated with pain and morbidities inherent to the procedure. Three of our patients who underwent open lung biopsy had prolonged air leak and another patient had surgical wound infection. We had only three cases of pneumothorax during TBLB, which were managed with Inter-costal Drainage (ICD). The ICD tubes could be removed in less than 5 days in all the three. There were four bleeding episodes during TBLB, all of which were controlled with conservative bronchoscopic treatment. All the other patients did not have any morbidity secondary to the procedure. We did a post biopsy chest radiograph only if the patient had pain, progressive hypoxia or a discrepancy in breath sounds. Routine use of chest radiographs after transbronchial biopsy is not cost-effective in asymptomatic patients and transbronchial biopsy with fluoroscopy has not been associated with a significantly lower incidence of pneumothorax than biopsy performed without fluoroscopy [12,17,18]. Other complications that have been reported include mediastinal and subcutaneous emphysema, none of which occurred in our patients.
Limitation
Though we included all consecutive DPLD patients, a larger sample size would have helped in assessing the diagnostic yield among specific DPLD subtypes. A follow-up evaluation of all patients also could not be done to assess the treatment response.
Conclusion
In DPLD patients with clinicoradiological discordance, TBLB should be utilized early in the diagnostic flow chart as an easily available and effective diagnostic modality before considering open lung biopsy. As shown in our study which indeed can be extrapolated to settings without facilities for a full-fledged advanced bronchoscopy suite, an unguided TBLB if properly performed is safe. Final diagnosis should be reached only after following a multidisciplinary approach. The recently described cryobiopsy technique for increasing TBLB yield with acceptable complications, especially in idiopathic interstitial pneumonia, could reduce to a great extent the issues of representative tissue sample in the near future. With advancements in technology, the future would be on implementing immunohistochemistry, molecular biology and microarray for diagnosis, even on tiny samples obtained from the TBLB to increase the diagnostic yield and reduce the need to refer for surgical lung biopsy.
(ILD-Interstitial Lung Disease, IIP-Idiopathic Interstitial Pneumonia, NSIP-Non Specific Interstitial pneumonia, UIP-Usual interstitial Pneumonia, HP- Hypersensitivity Pneumonitis, COP-Cryptogenic Organising Pneumonia, PAP-Pulmonary Alveolar Proteinosis, GGO-Ground Glass Opacity, C/c-Chronic).