Interference of Vaccine Derived Polio Viruses with Diagnosis of Enteroviral Diseases in Neonatal Period
Mohammad Saeed Sasan1, Alireza Ataei Nakhaei2, Abdolvahab Alborzi3, Mazyar Ziyaeyan4
1 Associate Professor, Department of Paediatrics, Mashhad University of Medical Sciences, Imam Reza Hospital, Mashhad, Iran.
2 Assistant Professor, Department of Paediatrics, Mashhad University of Medical Sciences, Imam Reza Hospital, Mashhad, Iran.
3 Professor, Department of Paediatrics, Shiraz University of Medical Sciences, Namazi General Hospital, Shiraz, Iran.
4 Professor, Alborzi Clinical Microbiology Research Center, Shiraz University of Medical Sciences, Namazi General Hospital, Shiraz, Iran.
NAME, ADDRESS, E-MAIL ID OF THE CORRESPONDING AUTHOR: Dr. Mohammad Saeed Sasan, Mashhad University of Medical Sciences, Imam Reza Hospital, Mashhad, Iran.
E-mail: sasanms@mums.ac.ir, ziyaeyan@gmail.com
Introduction
Enteroviruses (EV) are a common cause of neonatal sepsis especially at the junction of summer and fall.
Aim
This study was planned to find the frequency of Enteroviral (EV) sepsis among neonates with clinical sepsis.
Materials and Methods
This is a prospective descriptive study. Rectal and pharyngeal swab samples were taken from all neonates with clinical sepsis and a control group of neonates with simple jaundice. EV was confirmed by both cell culture and RT-PCR. Anti polio antiserum was used to differentiate Polioviruses from Non Polio EVs (NPEV).
Results
We had 67 neonates with clinical sepsis and 31 cases of simple jaundice during 105 days. NPEVs were isolated from 2 cases (2.9%) of the sepsis arm and one neonate (3.2%) of the jaundice group. Polio virus was isolated from 16.2% and 15.3% of OPV recipients in the sepsis and jaundice group respectively.
Conclusion
Enteroviruses were not a common cause for neonatal sepsis in Nemazi hospital at the time of this study. OPV vaccinated neonates commonly pass the vaccine virus in their pharynx and stool which can be mistaken with NPEV.
Enterovirus, Neonate, Sepsis
Introduction
Enteroviruses (EV) are a common cause of neonatal sepsis especially at the junction of summer and fall. The aim of this study was to find the frequency of enteroviruses in neonates who were admitted for sepsis work up in Nemazi hospital (Iran. Shiraz).
Materials and Methods
During an observational study from August 2001 up to November 2001, rectal and pharyngeal swab samples were taken from all neonates who were admitted in neonatology ward of Nemazi Hospital, with clinical diagnosis of sepsis, and also from otherwise healthy neonates who were admitted for neonatal jaundice (The reason for choosing the control group was to find how many of the EV positive neonates are symptomatic). The samples were transmitted in transfer tubes to cell culture media and for those with cytopathogenic effect (CPE) typical for EVs, RT-PCR for Enteroviruses was done. Anti polio antiserum was used to differentiate Polioviruses from Non Polio Enteroviruses (NPEV) on cell culture media.
Results
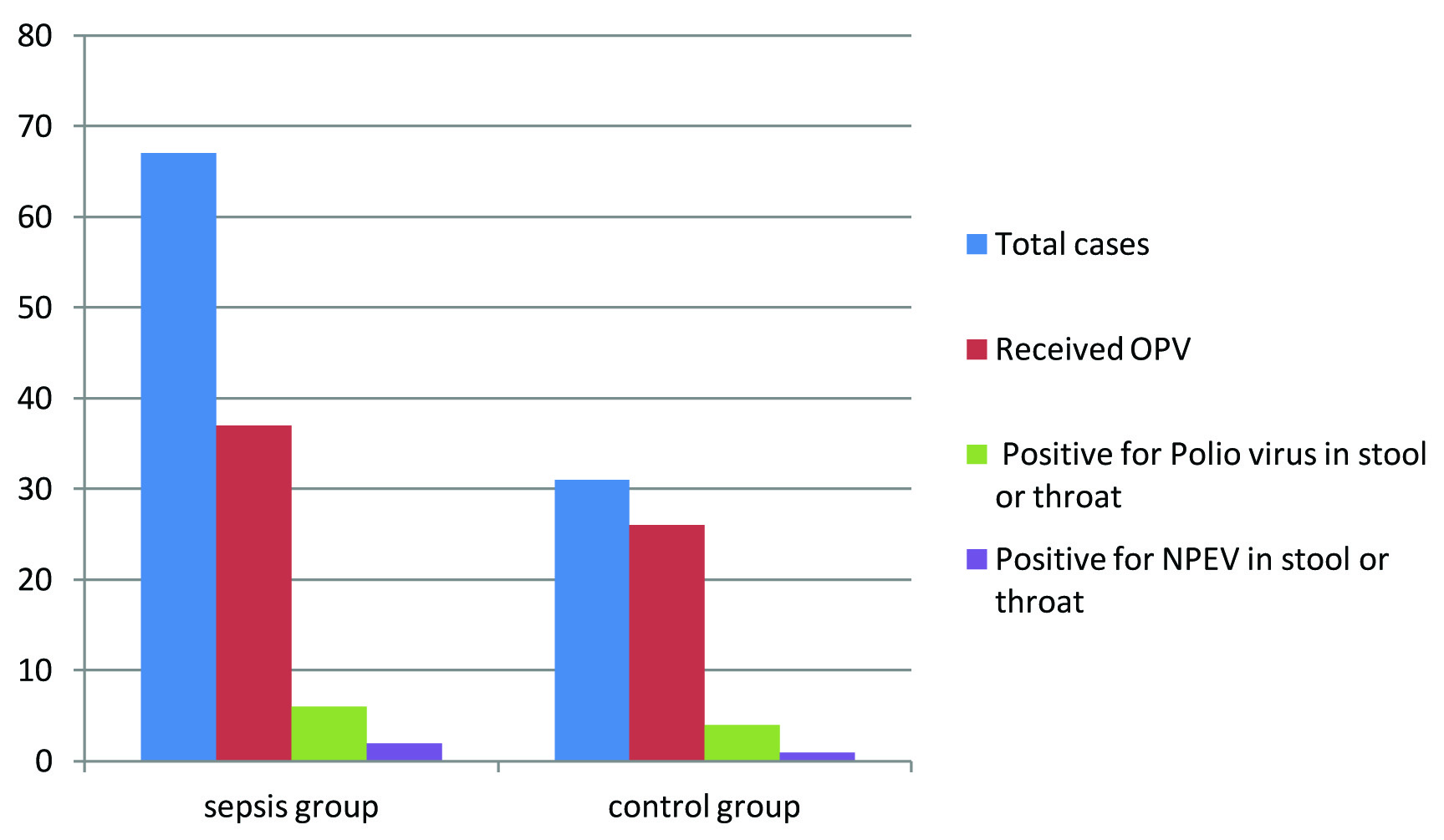
During 105 days, 67 neonate with clinical diagnosis of sepsis (mean age 16 days) and 31 non septic neonates with jaundice (mean age 9.1 days) were entered to the study. EV was isolated from 13.2% (13 cases) of all neonates, but just 3 (23%) of the EV isolates were NPEV. All of the positive EV samples were from rectal swabs except one case in which both the rectal and pharyngeal sample were positive.
In the sepsis group 37 and in the jaundice arm 26 neonates had received Oral Polio Vaccine (OPV) which Polio virus was isolated from 6 (16.2%) and 4 (15.3%) of them respectively. The mean age of polio virus positive cases was 16 days (2-27 days). None of the non vaccinated neonates were positive for Polio viruses. NPEV was isolated from 2 cases (2.9%) of the sepsis arm and one neonate (3.2%) of the jaundice group [Table/Fig-1].
Frequency of poliovirus and non polio enteroviruses isolation among neonates in shiraz.
Discussion
Sepsis is one of the leading causes of admission in the neonatal period, a bacterial pathogen is found in less than 20% of sepsis work ups in the first 2 month of life [1]. EVs are one of the most common causes of neonatal nonspecific febrile illnesses, accounting for approximately half of the hospital admissions for ruling out sepsis [2] The rate of viral pathogens in febrile infants (1 to 90-day-old) is 3 times of bacteria [3]. In Netherland EVs were detected in 57% of neonates (median birth weight: 3600) who were admitted for clinical sepsis in the paediatrics ward, while bacterial blood culture was not positive in any of them [4]. In a study among 139 neonates with culture negative sepsis (from Kuwait) EVs were detected in 24% of serum samples by PCR [5]. Jordán from Spain has reported the aetiology of fever in 72 febrile neonates for whom a final cause could be found. Bacterial infections were found in 62 patients (including 57 urinary tract infection, 3 sepsis and two cases of meningitis), while EVs (by PCR) were positive in 10 neonates [6]. Verboon has reported the retrospective results of viral infections in a NICU (in Netherlands) during a 12-year period. A viral infection was confirmed in 1% of 5396 admitted infants, EV and parechovirus (39%) respiratory syncytial virus (29%) and rotavirus (10%) were the most common viral pathogens in this study [7]. In a group of 75 premature (<32 weeks) neonates who were followed for 4 weeks following admission to a NIC in Sydney Australia, viral RNA or DNA (by multiplex PCR) was detected in 5.7% of stool samples, the culprit viruses were norovirus (1.9%), enterovirus and herpes simplex each one (1.2%), cytomegalovirus (0.7%), Epstein-Barr virus (0.5%) and rotavirus (0.2% [8]). In Mashhad we have shown that EVs are by far the most common cause of meningitis in the neonatal period and EV meningitis was 13 times more common than bacterial meningitis [9].
Oropharyngeal and stool samples for diagnosis of EV diseases have several limitations, including positive results from OPV and also prolonged carriage from a previous EV infection. However, most non-polio EVs in stool is aetiologically related to the current illness. A study in Estonia which evaluated the occurrence of enteroviruses in feces samples of 55 infants before and after OPV vaccination showed that 5.1% of non-vaccinated neonates had positive stool culture for EV (all non polio EV), while at the 6th month of life (after at least two doses of OPV) 67% of stool samples contained EV (all polioviruses) [10]. In South Africa 30% of stool samples from healthy (vaccinated) children (0-18 month) tested positive for polioviruses. Polio virus excretion generally stopped by the end of the second week after each vaccination [11]. In Bangladesh 17.6% of the stool samples from infants were positive for polioviruses up to 25 days after vaccination [12]. This rate was 15%-16% in our study, although all the cases in our group were not in the first 2 weeks after vaccination.
Conclusion
Enteroviruses were not a common cause for neonatal sepsis in Nemazi hospital at the time of this study. Isolation of NPEV from pharynx or feces is not specific for EV sepsis and asymptomatic passing of virus occurs. OPV vaccinated neonates commonly pass the vaccine virus in their pharynx and stool which can be mistaken with NPEV.
[1]. Bressan S, Predicting severe bacterial infections in well-appearing febrile neonates: laboratory markers accuracy and duration of feverPaediatr Infect Dis J 2009 29(3):227-32. [Google Scholar]
[2]. Hawkes MT, Vaudry W, Nonpolio enterovirus infection in the neonate and young infantPaediatr Child Health 2005 10(7):383-84. [Google Scholar]
[3]. Byington CL, Serious bacterial infections in febrile infants 1 to 90 days old with and without viral infectionsPaediatrics 2004 113(6):1662-66. [Google Scholar]
[4]. Verboon-Maciolek MA, Diagnosis of enterovirus infection in the first 2 months of life by real-time polymerase chain reactionClin Infect Dis 2003 37(1):1-6. [Google Scholar]
[5]. Ahmad S, Dalwai A, Al-Nakib W, Frequency of enterovirus detection in blood samples of neonates admitted to hospital with sepsis-like illness in KuwaitJ Med Virol 2013 85(7):1280-85. [Google Scholar]
[6]. Jordan I, Severe enterovirus disease in febrile neonatesEnferm Infecc Microbiol Clin 2009 27(7):399-402. [Google Scholar]
[7]. Verboon-Maciolek MA, Clinical and epidemiologic characteristics of viral infections in a neonatal intensive care unit during a 12-year periodPaediatr Infect Dis J 2005 24(10):901-04. [Google Scholar]
[8]. Naing Z, Prevalence of viruses in stool of premature neonates at a neonatal intensive care unitJ Paediatr Child Health 2013 49(3):E221-26. [Google Scholar]
[9]. Ghabouli Shahroodi MJ, Ghazvini K, Sadeghi R, Sasan MS, Enteroviral Meningitis in neonates and children of Mashhad, IranJundishapur J Microbiol 20167 9(5):e19955 [Google Scholar]
[10]. Salur L, Enterovirus infections in young infants: are children still protected by maternal antibodies?Hum Vaccin 2011 7(9):966-71. [Google Scholar]
[11]. Pavlov DN, Poliovirus vaccine strains detected in stool specimens of immunodeficient children in South AfricaDiagn Microbiol Infect Dis 2006 54(1):23-30. [Google Scholar]
[12]. Taniuchi M, Kinetics of poliovirus shedding following oral vaccination as measured by quantitative reverse transcription-PCR versus cultureJ Clin Microbiol201453(1):206-11. [Google Scholar]