Jaundice, also known as icter, is one of the most common neonatal problems. This condition can be seen in approximately 60% of full-term neonates and 80% of pre-term newborn during the first week after birth [1,2]. Jaundice appears clinically in neonates when bilirubin level is higher than 85μmol/L(5mg/dL). However, a bilirubin level of 34μmol/L (2mg/dL) is considered as an indication of jaundice in adults [3].
Dermal jaundice is initially seen in the face of neonates with an increase in the bilirubin level, this condition progresses caudally to the trunk and then to the extremities [4]. Dermal jaundice is commonly present in neonates, affecting over half of all newborns (50-60%) in the first week of life [5]. The incidence rate of severe neonatal jaundice in some developing countries is 100 times higher than developed countries [6,7].
Under normal circumstances, physiological and pathological jaundice would terminate within two weeks after its presentation [8]; however, in upto 15% of newborns, jaundice persists for more than 14 days [9]. Prolonged neonatal jaundice could be caused by factors such as hypothyroidism, congenital deficiency of glucuronyl transferase enzyme, breastfeeding, haemolysis, intestinal obstruction, pyloric stenosis and Gilbert’s syndrome [10,11].
Many studies have indicated the pathogenetic role of Gilbert’s syndrome in neonatal hyperbilirubinemia [12,13]. Gilbert’s syndrome refers to molecular changes in one specific isoform of UDP-glucuronosyl transferase (UGT1A) gene [14,15]. UGT1A1 gene polymorphism and Gilbert’s syndrome are widely ignored, despite their prevalence at the community level.
In fact Gilbert syndrome is the result of a genetic mutation in the promoter region of a gene for the enzyme UGT1A (one of the enzymes called UGT glucuronosyl transferases that are important for bilirubin metabolism). The gene is located on chromosome 2 [16]. Other types of mutations in the same gene cause the Crigler-Najjar syndrome, which is a more severe and dangerous form of hyperbilirubinemia (high bilirubin in the blood). The relationship between this syndrome and haemolytic anaemia increases the risk of hyperbilirubinemia and cholestasis [17].
The role of Gilbert’s syndrome in neonatal hyperbilirubinemia (bilirubin level > 223mmol/L during the first 7 days of life) has been evaluated in several studies and genotype frequencies of Gilbert’s syndrome have been investigated [18–20].
In infants with Gilbert’s syndrome, it takes longer to reach the normal bilirubin level. These results confirmed that Gilbert’s syndrome is one of the contributing factors for neonatal hyperbilirubinemia, although other factors are also involved in the diagnosis of jaundice. Slower decrease of bilirubin level for A (TA) 7TAA in homozygous neonates indicates that Gilbert’s syndrome is an important factor for determining prolonged jaundice [21]. Considering the importance of neonatal protracted jaundice and lack of studies on this subject, we aimed to determine the frequency of UGT1A1 polymorphism and its importance in the incidence of prolonged jaundice by ruling out other underlying causes.
Materials and Methods
This case–control study, conducted during October 2012 to November 2014, included 87 term infants (study group), with no specific risk factors for jaundice, hypoxia, haemolysis or sepsis. The subjects suffered from prolonged jaundice (bilirubin level > 10mg/dL) for more than 14 days, without presenting with any risk factors for haemolysis (such as ABO incompatibility, defects in G6PD enzyme and negative Coombs’ test results) or sepsis [3]. Therefore, they were admitted to paediatric clinic.
The exclusion criteria were as follows: severe underlying diseases, specific risk factors for jaundice hypoxia, haemolysis or sepsis; and use of phenobarbital or other medications by their respective mothers. The control group consisted of 81 infants without jaundice, who were referred to Taleghani Childrens Hospital. The two groups were matched in terms of age and gender. After obtaining consents from the parents, 3ml blood samples were collected from each infant.
Demographic data including age, gender, ethnicity and type of disease were recorded in a questionnaire and 3 ml blood samples were obtained from all subjects. Blood samples were kept in a Falcon tube (containing EDTA solution as the anticoagulant) and were stored at 20°C until DNA Extraction and Genotyping.
DNA Extraction
Five cc of blood was taken from each infant, using EDTA containing tubes. The genomic DNA was extracted, using phenol-chloroform method, with some modifications in the standard protocol [22]. The isolated DNA was kept in 1mL of Tris-HCl at -20°C. Specific primers were designed to detect the UGT1A1 polymorphism at G71R position, with an initial denaturation at 95°C for 10 min, 30 cycles of denaturation at 95°C for 1 min, annealing at 64°C for 1 min, extension at 72°C for 1 min and final extension at 72°C for 5 min PCR-CTPP was conducted [23].
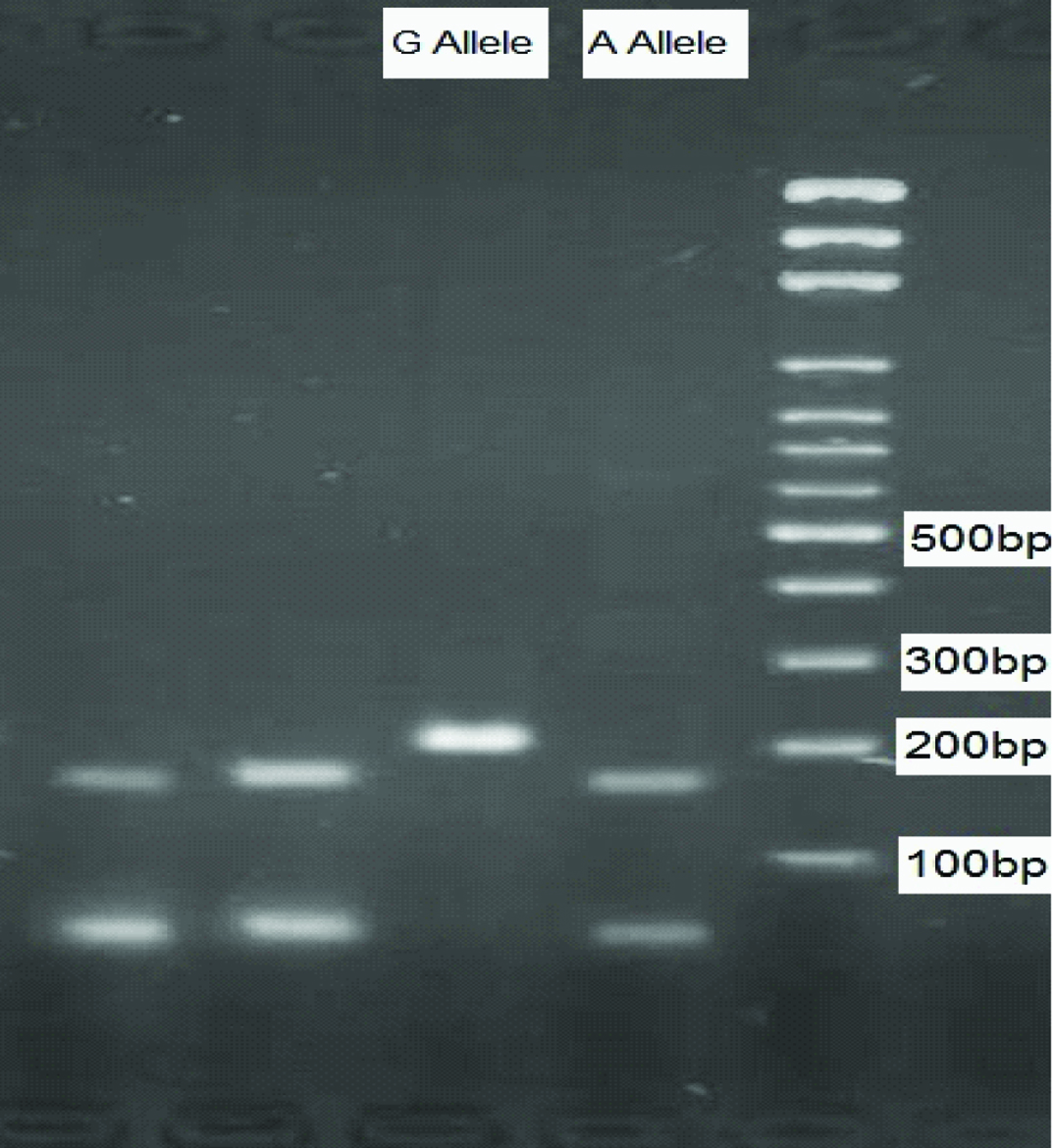
The used primers were as follows: F1: 5’-GGA AGA TAC TGT TGA TCC CAG TG-3’; R1: 5’-CGT CTT CAA GGT GTA AAA TGC TCC-3’; F2: 5’-GCC TCG TTG TAC ATC AGA GAC A-3’; and R2: 5’-GTA AGT GGG AAC AGC CAG AC-3’. The amplified DNA fragments were 159bp for the Gly(G) allele, 202bp for the Arg(A) allele, and 309bp for the common band. The amplified DNA was visualized on 2% Agarose gel, using DNA Green Viewer staining, as demonstrated in [Table/Fig-1].
The bands on Agarose gel.
Statistical Analysis
Data were evaluated by SPSS version 16 and the mean values of quantitative data were calculated by t-test. To compare the qualitative data (e.g., the frequency of genotypes and alleles), Chi-square and Fisher’s-exact test were applied. Bonferroni correction method was applied in case of any modifications.
Results
Among all infants (n=168), the mean bilirubin level was 10.68 and the mean age at disease onset was 16.82 days. [Table/Fig-2] shows the characteristics of study and control groups.
| Case | Control |
|---|
| Male | 87.3% | 74.6% |
| Female | 12.7% | 25.4% |
| Male/female ratio | 6.87 | 2.94 |
Allele and Genotype Frequency
No significant differences were observed between the study and control subjects in terms of genotype frequency. Among 87 cases in the study group, 73(84%) were male and 14 (16%) were female. Among 81 subjects in the control group, 52 (64%) were male and 29 (36%) were female.
The genotypic frequencies of the study group were as follows: A/G (83.9%), A/A (5.7%) and G/G (10.3%). Also, the corresponding values in the control group were as follows: A/G (84%), A/A (3.7%) and G/G (12.3%) [Table/Fig-3].
The frequency of UGT1A1 gene polymorphism in the study and control groups.
| Type of polymorphism | Study group | Control group | Total |
|---|
| A/A | 5(5.7%) | 3(3.7%) | 8(4.8%) |
| A/G | 73(83.9%) | 68(84%) | 141(83.9%) |
| G/G | 9(10.3%) | 10(12.3%) | 19(11.3%) |
The analysis showed no significant association between the incidence of prolonged jaundice and UGT1A1 polymorphism (p=0.772). The odds ratio of AA/GG polymorphism was 1.85 in the study group and 1.85 in the control group (95% CI = 0.341-10.04). Risk of prolonged jaundice in newborns with A/A allele was 1.32 times higher than cases with GG allele; however, the difference was not statistically significant (RR=1.3 0.645 -2.7) (p=0.475).
The ratio of A/A to A/G polymorphism was 1.55 folds higher in the study group, compared to the control group (OR=1.55, 95% CI = 0.35-6.7). The risk of jaundice in newborns with A/A allele was 1.2 times higher than subjects with A/G genotype; however, there was no significant difference (p>0.05). In total, 5.7% of subjects in the study group and 3.7% of infants in the control group were homozygous for A/A genotype, which indicated that cases with prolonged jaundice (over 14 days) were homozygous for A/A genotype; however, this difference was not statistically significant (p=0.18).
The frequency of UGT1A1 polymorphisms in male subjects was evaluated. In total, 80% of males in the study group and 84.3% of males in the control group had A/G genotypes; however, the difference was statistically not significant. Overall, 8.6% and 3.9% of males in the study and control groups had A/A genotypes, respectively. There was a statistically significant difference between the two groups (p=0.03). Also, 11.4% and 11.8% of males in the study and control groups had G/G genotypes, respectively; however, there was no significant difference (p>0.05).
In total, 92.3% and 83.3% of females in the study and control groups had A/G genotypes, respectively. Also, 7.7% and 16.7% of females in the case and control groups had G/G genotypes, respectively [Table/Fig-4]. The current findings showed that 8.6% and 3.9% of males in the study and control groups were homozygous for A/A genotypes in UGT1A1 G71R polymorphism; nonetheless, none of the females were homozygous for this allele. On the other hand, 7.7% of females in the study group and 16.7% of females in the control group had G/G alleles; this finding was statistically significant (p=0.03).
Frequency of UGT1A1 polymorphism among male and female subjects in the study and control groups.
| Groups | | A/A | A/G | G/G |
|---|
| Males | Study | 8.6% | 80% | 11.4% |
| Control | 3.9% | 84.3% | 11.8% |
| Total | 6.6% | 81.8% | 11.6% |
| Females | Study | 0% | 92.3% | 7.7% |
| Control | 0% | 83.3% | 16.7% |
| Total | 0% | 86.5% | 13.5% |
Discussion
Neonatal jaundice may result in life threatening disorders and could lead to neonatal morbidity and mortality. In this study, we examined the association between UGT1A1 polymorphisms at G71R region. Our findings showed that the association between prolonged neonatal jaundice and UGT1A1 G71R polymorphism was not statistically significant (p=0.772). However, a significant association was observed between prolonged jaundice in male infants and UGT1A1 G71R polymorphism (p=0.03). In our study, although G71R mutation among A/A homozygous infants in the study group differed from the control group by 2%, the difference was statistically not significant. Our findings were quite similar to those reported by Agrawal and colleagues [24].
Prachukthum et al., studied genetic polymorphisms in Thai neonates with hyperbilirubinemia [25]. They found a noticeable difference between male infants in the study and control groups in terms of A/A homozygosity in UGT1A1 G71R polymorphism; in females, no homozygous cases for A/A were reported; these findings were not consistent with the current results. The significant difference between the two genders could explain the relation between UGT1A1 polymorphism and gender. Moreover, Yusoff et al., evaluated the prevalence of 7TAA, G71R, and G493R mutations of UGT1A1 gene in icteric newborns in a Malaysian population [26]. In general, the frequency of 7TAA was higher than G71R and G493R. Their study showed that UGT1A1 nucleotide 211 polymorphism is more prevalent in East Asian societies, whereas in our study, despite the high frequency of UGT1A1 polymorphism, no significant relationship was found between the polymorphism and jaundice.
Another study by Mauro et al., showed that among every 17 infants with prolonged jaundice, 16 had at least one UGT1A1 mutation [27]. In general, seven patients were homozygous for G71R; this also confirms the importance of this type of polymorphism and its association with jaundice in East Asian regions. Our findings were not indicative of any association between prolonged jaundice and G71R; this might indicate the lower significance of UGT1A1 G71R polymorphism in Iran.
Yoshihiro et al., reported that the frequency of mutated allele in the hyperbilirubinemia group was significantly higher than the control group [28]; however, we could not find an association between prolonged jaundice and G71R polymorphism. Huang et al., studied the relationship between bilirubin UDP-glucuronosyl transferase 1A1 gene and neonatal hyperbilirubinemia [29]. They suggested that paediatricians should closely follow hyperbilirubinemic infants, who carry homozygous 211 G to A variation in UGT1A1 gene.
Although the present study findings indicated that the cases with prolonged jaundice were homozygous for A/A, this difference was not statistically significant. Huang et al., in another study showed that Chinese ethnic origin was an independent risk factor for hyperbilirubinemia [30], while we did not find any association between genotypic frequency and hyperbilirubinemia.
Limitation
Find and collecting infants with gilbert’s syndrome and take consent from their parents.
Conclusion
The present study results showed no association between prolonged jaundice and UGT1A1 polymorphism. However, an association between prolonged jaundice and UGT1A1variation in male infants was seen. For confirming the obtained results, further studies on a larger population are required. Taken together, differences observed in our study could be clarified by the genetic background of the Iranian population (such as consanguineous marriage) and different ethnic groups (e.g., Fars, Turkmen and Sistani) among the study population.