Evaluation of Fluid Absorbency of Retraction Cords after Immersing in Two Retraction Medicaments – An In-vitro Study
Gautam Vishnubhotla1, Sreeramulu Basapogu2, Rajeev Kumar Reddy Karnati3, Pradeep Prabhu Dasari4, Mani Victor Thommandru5, Mohana Bindu Bethu6
1 Postgraduate Student, Department of Prosthodontics, GDCH, Hyderabad, Telangana, India.
2 Associate Professor and Incharge, Department of Prosthodontics, GDCH, Hyderabad, Telangana, India.
3 Professor, Department of Prosthodontics, GDCH, Hyderabad, Telangana, India.
4 Postgraduate Student, Department of Prosthodontics, GDCH, Hyderabad, Telangana, India.
5 Postgraduate Student, Department of Prosthodontics, GDCH, Hyderabad, Telangana, India.
6 Postgraduate Student, Department of Prosthodontics, GDCH, Hyderabad, Telangana, India.
NAME, ADDRESS, E-MAIL ID OF THE CORRESPONDING AUTHOR: Dr. Sreeramulu Basapogu, Associate Professor, Department of Prosthodontics, Government Dental College and Hospital, Afzalgunj, Hyderabad-500012, Telangana, India.
E-mail: drsreeramulub@gmail.com
Introduction
Dry retraction cords help to displace the gingiva and also to absorb the gingival crevicular fluid and saliva to maintain a dry field. When used along with medicaments whether these medicaments help to improve the absorption of fluid or affect the fluid absorption by decreasing the efficiency of the retraction cord is unknown.
Aim
The aim of the study was to know the effect of various medicaments on the fluid absorbency of the retraction cords and also, to know whether the thickness of the retraction cords influences it’s fluid absorbency.
Materials and Methods
A total of 90 samples of 5cm length retraction cords were taken. Cords were divided into 30 samples for each cord thickness of 0, 1 and 2. Of these 30 samples, 10 samples were used to measure dry weight (Group I), 10 samples were immersed in 15.5% ferric sulfate (Group II) and remaining 10 samples were immersed in 10% aluminium chloride (Group III) for a period of 20 minutes. The excess medicament was removed by blotting paper. Initial weight was recorded. Following this, five cords from each group were immersed in plasma solution and remaining in artificial saliva for 10 minutes. Then these were taken out and measured. The amount of the fluid absorbed was determined by subtracting the weight before fluid immersion (weight after immersion in test medicament) from the weight after fluid immersion (weight after immersion in plasma or artificial saliva). The study was analyzed through one-way ANOVA and Bonferroni post-hoc comparisons for pair wise differences.
Results
When immersed in medicaments, there is a significant difference in absorption of fluids (artificial saliva and plasma) between the untreated dry cord and cord treated with 15.5% ferric sulfate (p<0.05). But, there was no significant difference in fluid absorption between the dry untreated cord and cord treated with 10% aluminum chloride and between cords treated with 15.5% ferric sulfate and 10% aluminum chloride.
Conclusion
Ferric sulfate (15.5%) is a better medicament for absorption of fluid.
Artificial saliva, Electronic analytical balance, Human plasma
Introduction
Maintaining a dry field is very important in fixed partial denture therapy for making precise impressions. If dry field is not maintained the finish line recording in the impression will be hampered which will in turn affects the marginal integrity. Marginal integrity is one of the basic requirements for the restoration to maintain good periodontal health. Three types of finish lines exist. They are supra-gingival, equi-gingival and sub-gingival [1]. The entire impression process for fixed prosthodontics requires careful management of the soft tissue [2]. Fluid control is must for all three types of finish lines and retraction is must for sub-gingival preparation. An effective management of the fluid control in the gingival sulcus is needed for successful sub-gingival impression [3].
Various techniques and methods have been used to manage gingival tissues. They include: a) Mechanical methods; b) Mechanico-chemical methods; c) Rotary gingival curettage; d) Electro-surgery. Amongst the known retraction techniques, immersed or impregnated cord in haemostatic solution is widely used and it is also the most conservative method [1].
Materials used for gingival retraction should be effective, material should not cause significant irreversible tissue damage and should not produce potentially harmful systemic effects [4].
Various Medicaments Include: 1) 0.1% and 8% racemic epinephrine; 2) 100% alum; 3) 5% and 25% aluminum chloride; 4) ferric sub-sulfate (Monsel’s Solution); 5) 13.3% ferric sulfate and 15.5% ferric sulfate; 6) 8% and 40% zinc sulfate; 7) 20% and 100% tannic acid; 8) 45% negatol (condensation product of metacresol, sulfonic acid and formaldehyde) [5].
Aluminum chloride is a chemical used in concentrations of 5 to 25% [6]. When used at a higher concentration than 10%, it produces local tissue destruction. Application for 10 minutes into the sulcus is effective in tissue displacement and to control sulcular bleeding. It produces an inflammatory reaction similar to 8% racemic epinephrine and alum, with minimum systemic effects and no contraindications [7].
Ferric sulfate, an astringent generally used in concentration of 13 to 20%. However, at higher concentrations i.e., above 15%, it can lead to tissue irritation and post-operative root sensitivity. This causes transient ischemia which results in shrinkage of gingival sulcus. The sulcus rebounds quickly after the cord is removed, the impression therefore, should be made immediately. In addition, it also helps in controlling the seepage of Gingival Crevicular Fluid (GCF) [8].
This study deals with fluid absorbency analysis of retraction cords after soaking them in 10% aluminum chloride and 15.5% ferric sulfate. The fluids used here were artificial saliva (saliveze) and plasma (used because GCF composition is similar to plasma).
The aim of the present study was to know whether the thickness of retraction cord has an effect on fluid absorption, to evaluate the effect of medicaments (15.5% ferric sulfate and 10% aluminum chloride) on absorption of fluids and to evaluate which medicament is better among the two to absorb fluids.
Materials and Methods
This in-vitro study was conducted in Department of Prosthodontics and Implantology, Goverment Dental College and Hospital, Hyderabad, Telangana, India, from 2014 to 2015. The ethical clearance for this study has been obtained from the academic ethical clearance committee of Goverment Dental College and Hospital headed by Principal of the institution. The materials required were: 1) Retraction cords sizes 0, 1 and 2 (Ultrapak plain cord, Ultradent, South Jordan, U.S.A); 2) Retraction medicaments i.e., 15.5% ferric sulfate and 10% aluminum chloride (M.P. Sai Biomed, Mumbai, India); 3) Fluids used were artificial saliva (Saliveze, Wyvren Medical Ltd., Ledbury, U.K.), human plasma (G.V.K. Bio., Hyderabad, India); 4) Electronic analytical balance to measure weights (Sartorius, Sartorius India, Secunderabad, India); 5) Blotting paper (Yash Filters, Ahmedabad, India); 6) Pipette (Eppendorf India Ltd., Kilpauk, Chennai, India).
Each retraction cord was cut into 30 samples of 5cm length [Table/Fig-1]. Medicaments used were 15.5% ferric sulfate and 10% aluminium chloride [Table/Fig-2]. Of these 30 samples, 10 samples were used to measure dry weight. Of the remaining 20 samples of each thickness, 10 samples were immersed in 15.5% ferric sulphate and remaining 10 samples were immersed in 10% aluminum chloride. These were left immersed for a period of 20 minutes. The excess fluid was removed by blotting paper [Table/Fig-3] saturated in corresponding test solution held between thumb and index finger. Initial weight was recorded on electronic analytical balance [Table/Fig-4]. Following this, five cords from each group were immersed in plasma solution and remaining in artificial saliva solution [Table/Fig-5] for 10 minutes. Then these were taken out and weight was again measured. The amount of the fluid absorbed was determined by subtracting the weight before fluid immersion from the weight after fluid immersion. The amount of fluid absorbed in the dry retraction cord was obtained by subtracting the weight of dry cord from the weight of cord after fluid immersion (weight after immersion into test medicament). The amount of fluid absorption after medicament treatment was obtained by subtracting the weight after medicament immersion from the final weight after fluid immersion (weight after immersion into plasma or artificial saliva). Methodology of how the samples were divided into sub-groups is described in [Table/Fig-6].
Retraction cords cut into 30 samples.



Electronic analytic balance.

Artificial saliva and human plasma.

Shows methodology of how the samples were divided into sub-groups.
| Retraction Cords | Medicament | Groups | Sub-groups n=5 |
|---|
| 0 | Untreated/Dry | I 0 | Soaked in plasma |
| 0 | 15.5% Ferric Sulfate | II 0 |
| 0 | 10% Aluminium Chloride | III 0 |
| 0 | Untreated/Dry | I 0 | Soaked in artificial saliva |
| 0 | 15.5% Ferric Sulfate | II 0 |
| 0 | 10% Aluminium Chloride | III 0 |
| 1 | Untreated/Dry | I 1 | Soaked in plasma |
| 1 | 15.5% Ferric Sulfate | II 1 |
| 1 | 10% Aluminum Chloride | III 1 |
| 1 | Untreated/Dry | I 1 | Soaked in artificial saliva |
| 1 | 15.5% Ferric Sulfate | II 1 |
| 1 | 10% Aluminum Chloride | III 1 |
| 2 | Untreated/Dry | I 1 | Soaked in plasma |
| 2 | 15.5% Ferric Sulfate | II 1 |
| 2 | 10% Aluminum Chloride | III 1 |
| 2 | Untreated/Dry | I 1 | Soaked in artificial saliva |
| 2 | 15.5% Ferric Sulfate | II 1 |
| 2 | 10% Aluminum Chloride | III 1 |
Results
One-way ANOVA comparison was done between a dry cord, ferric sulfate and aluminium chloride at room temperature [Table/Fig-7]. The results of Bonferroni Post-hoc test show that there is a significant difference in absorbency between dry cord and ferric sulfate immersed cord. However, there was no significant difference between ferric sulfate and aluminium chloride immersed cords. Nevertheless the absorbency is more with ferric sulfate treatment [Table/Fig-8]. The results of present study show that the fluid absorbency with or without medicament increases as the thickness of the cord increases [Table/Fig-9,10].
The one-way ANOVA comparison showing the effect of absorption of fluids upon immersion in aluminium chloride and ferric sulfate and with dry cord at room temperature.
| N | Mean | SD | Std. error | 95% Confidence interval for mean | F | Sig. |
|---|
| Lower Bound | Upper Bound |
|---|
| Dry Cord [RT] | 30 | 68.846 | 19.554 | 3.57008 | 61.5450 | 76.1483 | 7.598 | 0.001 |
| 7 | 13 |
| Ferric Sulphate | 30 | 89.353 | 20.889 | 3.81387 | 81.5531 | 97.1536 |
| 3 | 43 |
| Aluminium Chloride | 30 | 77.296 | 20.961 | 3.82705 | 69.4695 | 85.1239 |
| 7 | 60 |
| Total | 90 | 78.498 | 21.944 | 2.31314 | 73.9027 | 83.0950 |
| 9 | 36 |
p-value is significant (0.001), RT- Room temperature
Bonferroni Post-hoc comparisons: This test was done to compare the weight of dry cord, the cord immersed in ferric sulfate, the cord immersed in aluminium chloride.
| Medicament | Medicament | Mean difference(I-J) | Std. error | Sig. |
|---|
| Dry Cord | Ferric Sulphate | -20.50667* | 5.28755 | 0.001 |
| Aluminium Chloride | -8.45000 | 5.28755 | 0.341 |
| Ferric Sulfate | Dry Cord | 20.50667* | 5.28755 | 0.001 |
| Aluminium Chloride | 12.05667 | 5.28755 | 0.075 |
| Aluminium Chloride | Dry Cord | 8.45000 | 5.28755 | 0.341 |
| Ferric Sulphate | -12.05667 | 5.28755 | 0.075 |
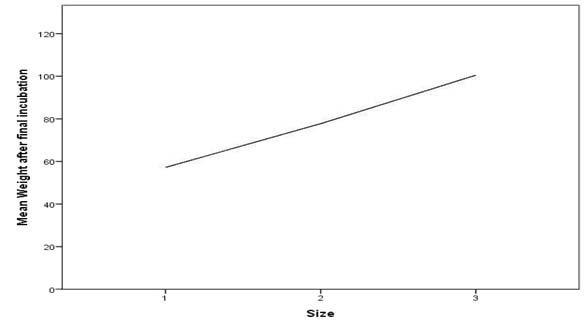
Spearman’s correlation test implies that there is a significant increase in weight of thread as the size of thread increases.
| | Size of cord | Weight afterfinalincubation |
|---|
| Size of cord | CorrelationCoefficient | 1.000 | 0.813**0.000 |
| Sig. (2-tailed) | - | 0.000 |
| Spearman’s rho | N | 90 | |
| Weight incubation | CorrelationCoefficientafter final | 0.813** | 1.000 |
| Sig. (2-tailed) | 0.000 | . |
| N | 90 | 90 |
** Correlation is significant at the 1% level (2-tailed).
The results of the present study shows that fluid absorbency with or without medicaments increases as the thickness of the cord increases.
With respect to treatment with 10% aluminium chloride, the cord which absorbed maximum fluid is size “2” in accordance with Spearman’s correlation test and the fluid which got absorbed highest was human plasma [Table/Fig-11,12].
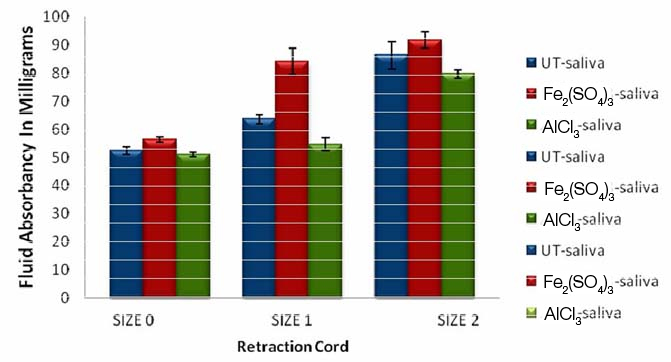
As the thickness of the cord increases the amount of saliva absorption also increases. Saliva absorption is more in a retraction cord immersed in 15.5% ferric sulfate [Fe2(SO4)3] compared to untreated cord and cord immersed in 10% aluminium chloride (AlCl3) in all thicknesses.
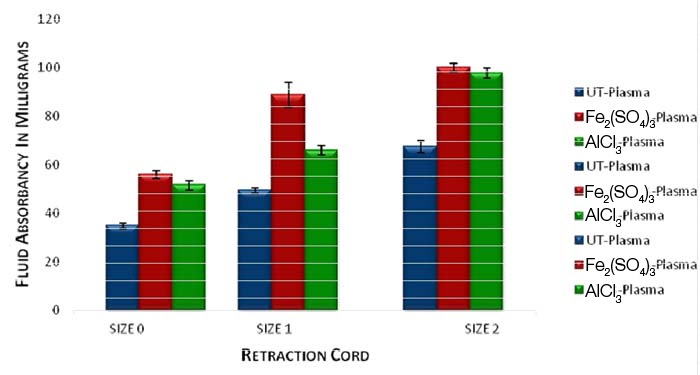
As the thickness of the cord increases the amount of plasma absorption increases. The amount of plasma absorption is more in a retraction cord immersed in 15.5% ferric sulfate compared with the cord immersed in 10% aluminium chloride and untreated dry cord.
With respect to treatment with 15.5% ferric sulfate, the cord which absorbed maximum fluid is size “2” in accordance with Spearman’s correlation test and the fluid which got absorbed highest was human plasma [Table/Fig-11,12].
Of the two medicaments used in the study, artificial saliva and plasma absorption was more with 15.5% ferric sulfate when compared with 10% aluminum chloride irrespective of cord thickness [Table/Fig-12]. The fluid which was highly absorbed was human plasma in both the medicaments [Table/Fig-11,12].
Discussion
The rationale behind the present study was to find out an effective mechanico-chemical retraction method with minimum systemic effects. Maintaining the marginal integrity of the tooth is one of the most important basic principles of tooth preparation [9]. From the periodontal point of view, it is preferable to place the gingival finish lines of restoration supra-gingivally [10], but studies show that sub-gingival margins do not cause gingival inflammation [11]. The dentist may be forced to place the margin sub-gingivally for aesthetics or other reasons like caries, existing restoration and when additional retention is required. Among various medicaments used as retraction agents, the one used in this study was 10% aluminium chloride and 15.5% ferric sulfate because they cause minimal tissue damage [6], is readily available and less systemic effects.
A study by Jokstad A revealed that the consistency of gingival retraction cord, twined or knitted, seems to be more important than the medicament when related to preference [12]. It is not surprising that the consistency of retraction cord has been associated with packing easiness and cord fraying. It is more difficult to explain why haemostasis, sulcus dilation, bleeding on removal, and dryness of sulci were rated better for knitted than twined retraction cords.
According to study done by Kumbuloglu O et al., proper hemostatic action is dependent on the amount of medicament solution absorbed by the cord during soaking, which is also dependent on length, thickness, structure, moistening properties of cord and length of soaking time [13]. Also for successful gingival retraction, soaking time in medicament is crucial for proper ingress of medicament into cord. An optimum time for soaking of retraction cords in medicaments is 20 minutes [14]. In current study, the retraction cords in the first group were incubated in their medicament solutions for 20 minutes before using, as recommended by Csempesz F et al., [15].
Fluid absorbing capacity of cords does not decrease when soaked in haemostatic solution like alum and aluminum chloride. However, study done by Garg SK et al., has proved that thicker retraction cords have better fluid absorbency than thinner cords irrespective of medicament used. Hence, it has been concluded that the amount of fluid absorbed increases in linear proportion with the size of cord [16].
Structurally Ultrapak cords have chain like interlocking loops which gives them an advantage of bending in any direction passively. When compared to conventional cords Ultrapak cords have superior medicament retention capacity of up to 2.5 times [17]. In order to find out the effect of cord diameter on absorbency of fluid three different sizes (0, 1 and 2) were selected. All the cords were of same length (5cm) and from same manufacturer.
Studies were done earlier to evaluate the chemical medicament absorption by the retraction cords (Runyan DA et al.,) [18]. This study aimed at determining whether the medicaments hamper or enhance or have no action on fluid absorbency of the knitted retraction cords. The retraction cords in addition to displacing the gingiva absorb moisture and fluids present in the sulcus to create a dry field for impression making.
The logic behind the selection of blood and saliva as immersion fluids was that we encounter these fluids during retraction and have to be cleared off for an acceptable impression. To simulate crevicular fluid in-vitro, human plasma was chosen as it contains proteins similar to crevicular fluid and blood [19]. To simulate saliva in-vitro, artificial saliva was chosen.
Clinical Implications: Fluid absorbency of the cords is important to maintain a dry field. Medicaments should not hamper this property of the retraction cords. So, this study helps in inferring that 15.5% ferric sulfate is a better medicament to increase the fluid absorbency of the retraction cords.
Limitation
The limitations of the current study include that 15.5% ferric sulphate is acidic. The plasma used in the study differs from GCF in a few high molecular weight proteins and the artificial saliva doesn’t constitute all the components in saliva.
The current study was planned in view of mechanico-chemical retraction methods being extensively used in day today clinical practice. Further there is a scope for research towards more effective and less cumbersome retraction methods.
Conclusion
Medicaments and retraction cords are used in mechanico-chemical means of retraction of gingiva. The study gives a conclusion that irrespective of the medicament used, with increase in thickness of the retraction cord the fluid absorption increases. Both the medicaments can be safely used along with retraction cords as they do not decrease the fluid absorbency of the retraction cords when placed in gingival sulcus. The medicament better among the two (15.5% ferric sulfate and 10% aluminum chloride) used in this study was ferric sulfate for absorption of both human plasma and artificial saliva.
p-value is significant (0.001), RT- Room temperature
** Correlation is significant at the 1% level (2-tailed).
[1]. Rosensteil SF, Land HF, Fujimoto J, Principles of tooth preparationContemporary fixed prosthodontics 2006 4th edSt. LouisMosby-An Imprint of Elsevier [Google Scholar]
[2]. Benson BW, Bomberg TJ, Hatch RA, Hoffman W, Tissue displacement methods in fixed prosthodonticsJ Prosthet Dent 1986 55(2):175-81. [Google Scholar]
[3]. Prasad KD, Hegde C, Agrawal G, Shetty M, Gingival displacement in prostho-dontics: A critical review of existing methodsJournal of Interdisciplinary Dentistry 2011 1(2):80-86. [Google Scholar]
[4]. Donovan TE, Gandara BK, Nemetz H, Review and survey of medicaments used with gingival retraction cordsJ Prosthet Dent 1985 53(4):525-31. [Google Scholar]
[5]. Felpel LP, A review of pharmacotherapeutics for prosthetic dentistry: Part IJ Prosthet Dent 1997 77(3):285-92. [Google Scholar]
[6]. Shaw DH, Krejci RF, Cohen DM, Retraction cords with aluminium chloride: Effect on the gingivaOper Dent 1980 5(4):138-41. [Google Scholar]
[7]. deGennaro GG, Landesman HM, Calhoun JE, Martinoff JT, A comparison of gingival inflammation related to retraction cordsJ Prosthet Dent 1982 47:384-86. [Google Scholar]
[8]. Gupta Gaurav, Sunil Kumar M.V, Rao Harikesh, Garg Pooja, Kumar Rajesh, Sharma Alok, Astringents in dentistry: A reviewAsian J Pharm Hea Sci 2012 2(3):428-32. [Google Scholar]
[9]. Gardner FM, Margins of complete crowns-Literature reviewJ Prosthet Dent 1982 48(4):396-400. [Google Scholar]
[10]. Richter WA, Ueno H, Relationship of crown margin placement to gingival inflammationJ Prosthet Dent 1973 30:156-61. [Google Scholar]
[11]. Shillinburg HT, Sumiya H, Whitsett LD, Richard J, Brakette SE, Principles of tooth preparation: Fundamental of Fixed Prosthodontics 1997 3rd edChicagoQuintessence Publishing Co. Inc [Google Scholar]
[12]. Jokstad A, Clinical trial of gingival retraction cordsJ Prosthet Dent 1999 81(3):258-61. [Google Scholar]
[13]. Kumbuloglu O, User A, Toksavul S, Boyacioglu H, Clinical evaluation of different gingival retraction cordsQuintessence Int 2007 38(2):e92-98. [Google Scholar]
[14]. Patel Pankaj, Vaishnav Kalpesh, Ganatra Chaitalee, Pujara Tapan, Evaluation of kinetic absorbency of three different medicaments by five different types of cordsJ Int Oral Health 2011 3(1):15-21. [Google Scholar]
[15]. Csempesz F, Vag J, Fazekas A, In vitro kinetic study of absorbency of retraction cordsJ Prosthet Dent 2003 89(1):45-49. [Google Scholar]
[16]. Garg SK, Garg S, Mittal S, Goyal S, Comparative evaluation of fluid absorbancy of retraction cords of different thickness after various medicament immersionsJ Interdiscip Dentistry 2012 2:30-34. [Google Scholar]
[17]. Fisher DW, Conservative management of the gingival tissue for crownsDent Clin North Am 1976 20:273-84. [Google Scholar]
[18]. Runyan DA, Reddy TG Jr, Shimoda LM, Fluid absorbency of retraction cords after soaking in aluminium chloride solutionJ Prosthet Dent 1988 60(6):676-78. [Google Scholar]
[19]. Curtis MA, Sterne JA, Price SJ, Griffiths GS, Coulthurst SK, Wilton JM, The protein composition of gingival crevicularfiuid sampled from male adolescents with no destructive periodontitis: Baseline data of a longitudinal studyJ Periodont Res 1990 25(1):6-16. [Google Scholar]