Accumulating evidence, over the last few years, points towards the critical role played by oxidant stress in causation of tissue damage accompanying various diseases. A disturbance in the pro-oxidant/anti-oxidant balance is, increasingly, being considered responsible for the oxidative damage to various cellular molecules such as lipids, proteins, carbohydrates and DNA [3–5]. Oxidative stress has also been reported to have an important role in the pathogenesis of Iron Deficiency Anaemia (IDA) [6–10].
Iron deficiency not only affects the production of haemoglobin but also of other iron containing proteins, viz. cytochromes, myoglobin, catalase (CAT) and peroxidase. Since, in iron deficiency states enzymes of the antioxidant defense system are functionally defective, the balance tends to get tilted towards free radicals triggering oxidative damage. However, data on oxidative and anti-oxidant defences in IDA is rather limited and often contradictory [6–8,11–26].
Researchers have reported that, iron therapy reverses both the weakening of anti-oxidant defenses as well as the concomitantly increased lipid peroxidation encountered in patients with IDA [4,11,16,17,19,22–24,26]. However, iron therapy per se has also been shown to increase the generation of free radicals leading to oxidative stress [14,18,25,27,28]. Further, very few studies in this regard have been conducted thus far on children [11,17,19–26,29]. The present study was, therefore, undertaken to study the levels of markers of oxidative stress and anti-oxidant status in children with IDA and to assess the effect of iron therapy on the same.
Materials and Methods
This prospective, hospital based study was a sub-study of a Randomized Controlled Trial entitled “Effect of iron supplementation on markers of oxidative stress and anti-oxidant status in children with IDA” (Clinical Trials Registry of India no.: CTRI/2010/091/001102, dated 05-08-2010) conducted between October 2009 to February 2011 in the Department of Paediatrics, Jawaharlal Nehru Medical College, Aligarh Muslim University, Aligarh, Uttar Pradesh on children between 1-12 years with IDA. The sub-study was conducted in two parts: in the first part, levels of a biomarker of oxidative stress {malondialdehyde (MDA)} and anti-oxidant enzymes {superoxide dismutase (SOD), catalase (CAT), glutathione Peroxidase (GPx)} were assessed and compared between 67 children with IDA and 31 non-anaemic controls; in the second part, the effect of oral iron on these markers was studied in a subset of anaemic children.
Children with IDA having haemoglobin (Hb) between 6-11gm% were enrolled in the study group. Those with fever within last four weeks, acute or chronic medical disorders, haemolytic anaemia or anaemia of chronic disease, intake of iron/vitamin/mineral supplements (including herbal drugs) within the last eight weeks, and recipients of blood/ blood products within the last six months were excluded. The control group comprised 31 apparently healthy age and gender matched non-anaemic children who were not on any medication, iron, vitamin, or mineral supplements (including herbal drugs) within the last eight weeks.
The study was approved by the Institutional Ethics Committee. An informed written consent (including consent for blood sampling) was obtained from parents/legal guardians of eligible children for participation in the study.
Sample size
The sample of 67 anaemic children for this sub-study was drawn from an original RCT (referred to earlier) exploring the effect of 2 different dose iron regimens in children with IDA. Of these, 35 subjects who received the daily, conventional dose of iron were assigned to the 2nd part (iron therapy trial) of the substudy.
Intervention (for the iron therapy trial)
All anaemic subjects enrolled in the 2nd part of the study were first de-wormed with albendazole in age appropriate dosage. These children were then administered oral iron (Syrup Sodium feredetate, elemental iron 33mg/5ml) in a dose of 6mg/kg (of elemental iron) in 2 divided doses, between meals, for eight weeks. The subjects were followed up, fortnightly, for eight weeks post-intervention. Compliance was checked and information obtained regarding any complaints/ adverse effects on each follow-up. Besides this, the parents were advised to contact the trial staff at any time in case of any adverse effects/significant complaints or doubts. Trial deviates included patients with IDA who developed congestive cardiac failure needing blood transfusion, intolerance to oral iron therapy, infections, diarrhoea or any other condition needing temporary discontinuation of iron therapy/ additional treatments. The control group received no intervention.
Sample Collection and Investigations:
Blood sampling, in subjects with IDA, was performed twice: once at baseline i.e., before the initiation of iron therapy, and finally one day after completion of 8 weeks of treatment. In the control group, samples were collected only once at enrolment. Two ml venous blood was collected aseptically in EDTA vials by peripheral venous phlebotomy for measurement of RBC indices Mean Corpuscular Volume (MCV), Mean Corpuscular Haemoglobin (MCH), Mean Corpuscular Haemoglobin Concentration (MCHC) and Red Cell Distribution Width (RDW) by Lab Life 3D haematological autoanalyser. Additionally, 5 ml venous blood was collected in iron free vacutainers for estimation of iron {colorimetric technique (Spinreact, Spain)}, total iron binding capacity (TIBC) {saturation – precipitation technique (Spinreact, Spain)}, ferritin {ELISA (Calbiotech Inc.)}, MDA (Philpot’s method) [30], SOD (McCord and Fridovich’s method) [31], GPx (Paglia and Valentine’s method) [32], and CAT (Aebi’s method) [33].
Statistical Analysis
Data was analysed using SPSS version - 17 for Windows Software. Continuous variables were expressed as means±standard deviation (SD), confidence intervals (95% CI), medians (interquartile range), frequency and range. The Shapiro-Wilk Test was used to assess normality of data. T-test was applied for normally distributed data: Independent samples t-test to compare means of a variable for 2 unrelated groups; Paired sample t-test to compare means of 2 variables (i.e., pre and post-iron therapy) within a group. Non-parametric tests were applied when the distribution of data was non-normal: Mann–Whitney test for comparisons between 2 unrelated groups; Wilcoxon signed-rank test to compare between paired samples within a group. The Bivariate correlations procedure (Pearson correlation) was used to compute the pair wise associations for a set of variables. The p-values < 0.05 were taken as significant.
Results
A total of 122 patients with suspected IDA were evaluated initially, of which 94 patients fulfilled the criteria for inclusion in the study. Sixty seven children whose parents gave consent were enrolled in the study (IDA) group. The control group included 31 non-anaemic children. Thirty-five subjects from the IDA group were later included in the iron therapy trial, all of whom completed the study as per protocol. The ages of subjects in IDA and control group ranged from 12 -144 months (mean, 40.45± 29.78; 95% CI: 33.19- 47.71) and 12- 120 months (mean, 49.45±29.85; 95 % CI: 38.50-60.40), respectively. Maximum number of anaemic patients belonged to 12 -36 months age group (50.75%), followed by 36.1- 72 months age group (38.81%). Majority (38.72%) of subjects in control group were between 36.1 -72 months of age. Males accounted for 70.23% and 70.97% of subjects in the study and control group, respectively. Baseline characteristics of the subjects are presented in [Table/Fig-1].
Baseline characteristics of patients with IDA and controls.
| Characteristic | IDA Group(n= 67) | Control Group(n=31) | Mann-Whitney U # | p-value |
|---|
| Age (months) | 40.45±29.78(95% CI: 33.19- 47.71) | 49.45±29.85 *(95% CI: 38.50- 60.40) | - | 0.168 |
| Male : Female | 47: 20 | 22: 9 | - | - |
| BMI | 16.30 (15.30-17.40) | 17.20 (14.40-18.9) | 879.50 | <0.001 |
| Hb (g/dl) | 7.60 (6.70-8.50) | 12.60 (12.3-13.2) | <0.001 | <0.001 |
| MCV (fl) | 62.40 (57.30-68.60) | 82.50 (78.6-88.4) | 14.50 | <0.001 |
| MCH (pg/cell) | 19.60±3.34(95% CI: 18.79- 20.42) | 23.76±1.85 *(95% CI: 23.09- 24.44) | - | <0.001 |
| MCHC(% Hb/cell) | 28.80 (25.70-30.20) | 33.50 (32.4-34.3) | 136.50 | <0.001 |
| RDW (CV) (%) | 16.24±1.38(95% CI: 15.90-16.57) | 13.96±0.73 *(95% CI: 13.69-14.23) | - | <0.001 |
| Serum iron (μg/dl) | 43.30±9.25(95% CI:41.04-45.55) | 71.76±9.80 *(95% CI: 68.16- 75.35) | - | <0.001 |
| TIBC (μg/dl) | 456.08±94.1(95% CI: 433.12-479.05) | 306.57±48.25 *(95% CI: 288.87- 324.27) | - | <0.001 |
| Transferrin saturation (%) | 9.80±2.69(95% CI: 9.15-10.46) | 23.88±4.73 *(95% CI: 22.14-25.62) | - | <0.001 |
| Serum ferritin (ng/ml) | 18.45±6.67(95% CI: 16.82- 20.08) | 100.58±19.10*(95% CI: 288.87- 324.27) | - | <0.001 |
*: Data expressed as mean±standard deviation (SD); Independent samples t-test used for comparisons.
#: Data expressed as median with Interquartile Range (IQR) given in parenthesis; Mann–Whitney test used for comparisons.
Levels of all the markers of anti-oxidant status, viz. SOD, CAT, and GPx were observed to be significantly lower (p<0.001) in patients with IDA as compared to controls [Table/Fig-2]. On the other hand, levels of MDA, a biomarker of oxidative stress, were significantly higher (p<0.001) in the IDA group.
Comparison between markers of oxidative stress and anti-oxidant status in patients with IDA and controls.
| Marker | IDA Group(n= 67)Median (IQR) | Control Group(n=31)Median (IQR) | Mann-Whitney U | p-value |
|---|
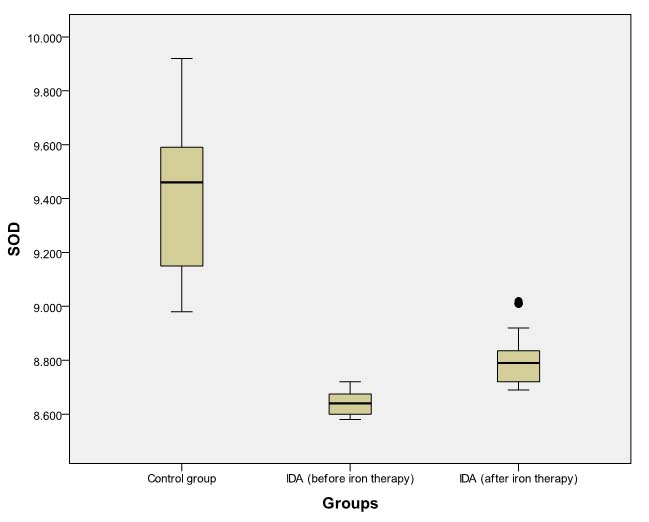
| SOD | 8.63 (8.60-8.66) | 9.46 (9.14-9.62) | 0.000 | <0.001 |
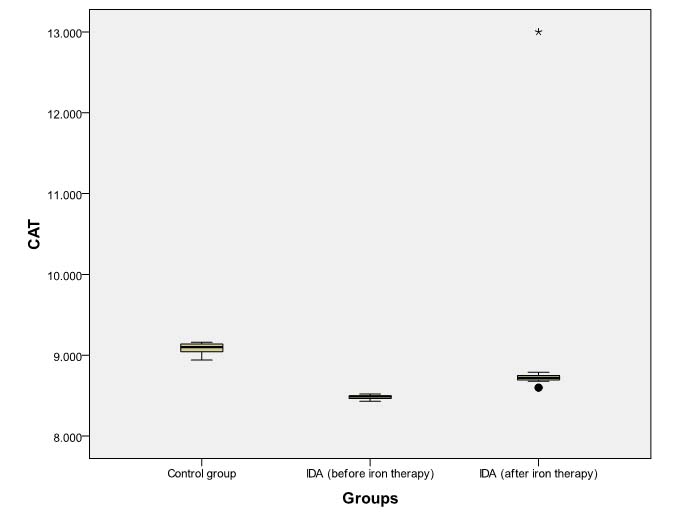
| CAT | 8.49 (8.46-8.50) | 9.10 (9.04-9.14) | 0.000 | <0.001 |
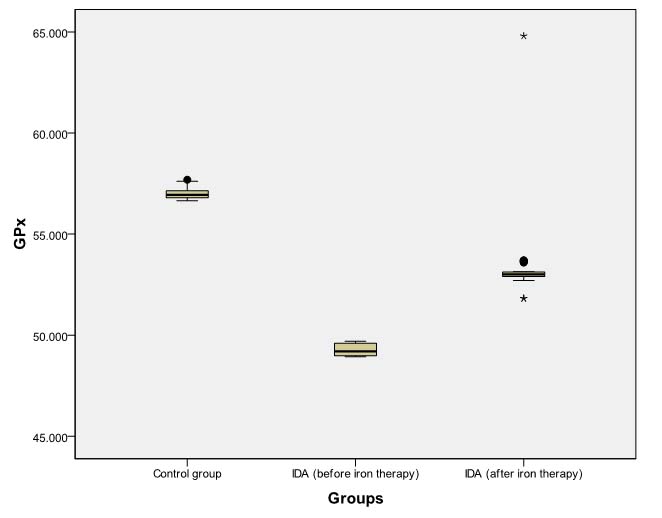
| GPx | 49.19 (48.99-49.60) | 56.94 (56.80-57.14) | 0.000 | <0.001 |
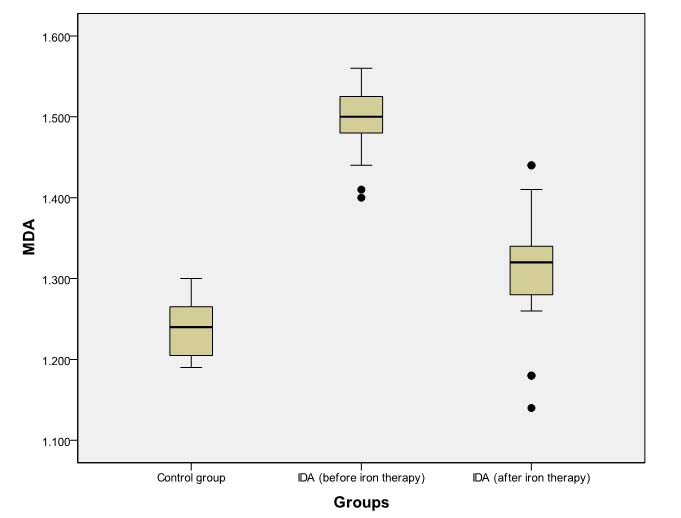
| MDA | 1.50 (1.48-1.52) | 1.24 (1.20-1.27) | 0.000 | <0.001 |
IQR: Interquartile range
Levels of Hb and markers of iron status (serum iron, transferrin saturation and serum ferritin) were observed to have a very strong and highly significant positive correlation (p<0.001) with levels of markers of antioxidant status (SOD, CAT, and GPx) [Table/Fig-3]. TIBC on the other hand, had a strong, highly significant (p<0.001) negative correlation with these markers. Levels of Hb and all markers of iron status (except TIBC) had a very strong, highly significant negative correlation (p<0.001) with MDA.
Correlation between haemoglobin, iron status and markers of oxidative stress and antioxidant status.
| Parameter | Correlation | SOD | CAT | GPX | MDA |
|---|
| Hb | Pearson correlation | 0.836 | 0.917 | 0.920 | -0.876 |
| p-value | <0.001 | <0.001 | <0.001 | <0.001 |
| Serum iron | Pearson correlation | 0.699 | 0.799 | 0.814 | -0.781 |
| p-value | <0.001 | <0.001 | <0.001 | <0.001 |
| TIBC | Pearson correlation | -0.612 | -0.656 | -0.644 | 0.629 |
| p-value | <0.001 | <0.001 | <0.001 | <0.001 |
| Transferrinsaturation | Pearson correlation | 0.796 | 0.883 | 0.882 | -0.851 |
| p-value | <0.001 | <0.001 | <0.001 | <0.001 |
| Serumferritin | Pearson correlation | 0.848 | 0.942 | 0.953 | -0.922 |
| p-value | <0.001 | <0.001 | <0.001 | <0.001 |
Results of the iron therapy trial:
After eight weeks of daily iron therapy, a highly significant rise (p<0.001) from baseline was observed in Hb, MCV, MCH, MCHC, serum iron, transferrin saturation, and serum ferritin levels [Table/Fig-4]. On the other hand, TIBC and RDW (CV) were observed to decline significantly (p<0.001). Despite treatment, the levels of Hb (p<0.001), serum iron (p=0.041) and serum ferritin (p<0.001) remained significantly lower in patients with IDA at eight weeks in comparison to baseline values of these parameters in the control group. However, the values of TIBC (p=0.168) and transferrin saturation (p=0.157) at eight weeks in the IDA group were similar to baseline control values.
Changes in haematological parameters and iron status after iron therapy in subjects with IDA.
| Parameters | Baseline(n=35) | After 8 weeks (n=35) | Mean* difference Z# | Z# | p- value |
|---|
| Hb (g/dl) | 7.40(6.60-8.00) | 11.40 (10.80-12.00) | - | -5.162 | <0.001 |
| MCV (fl) | 60.00(54.40-68.60) | 80.1(76.60-82.40) | - | -5.160 | <0.001 |
| MCH (pg/cell) | 18.30(16.20-18.30) | 24.60 (24.10-26.20) | - | -5.078 | <0.001 |
| MCHC (% Hb/cell) | 28.20(26.80-29.80) | 33.10 (32.20-33.80) | - | -5.144 | <0.001 |
| RDW-CV (%) | 16.60(15.60-17.30) | 14.1 (14.00-14.40) | - | -5.087 | <0.001 |
| Serum iron (μg/dl) | 43.30±9.25 | 65.27±9.28 | 23.87 | - | <0.001 |
| TIBC (μg/dl) | 456.08±94.10 | 301.11±49.23 | -163.34 | - | <0.001 |
| Transferrin saturation (%) | 9.80±2.69 | 22.22±4.69 | 13.07 | - | <0.001 |
| Serum ferritin (ng/ml) | 18.45±6.67 | 50.67±6.78 | 32.09 | - | <0.001 |
*: Data expressed as mean±standard deviation (SD); Paired samples t-test test used to compare before- and after-treatment changes.
#: Data expressed as median with interquartile range (IQR) given in parenthesis; Wilcoxon signed-rank test used to compare before- and after-treatment changes.
A highly significant rise (p<0.001) from baseline was observed in SOD, CAT, and GPx levels after eight weeks of daily iron therapy in subjects with IDA [Table/Fig-5]. On the other hand, MDA levels were observed to decline significantly (p<0.001) post-supplementation.
Changes in markers of oxidative stress and antioxidant status after iron therapy in subjects with IDA.
| Markers | BaselineMedian (IQR) | After 8 weeksMedian (IQR) | Z # | p-value |
|---|
| SOD | 8.64 (8.60-8.68) | 8.79 (8.72-8.85) | -5.088 | <0.001 |
| CAT | 8.49 (8.46-8.50) | 8.72 (8.69-9.75) | -5.164 | <0.001 |
| GPx | 49.20 (48.99-49.60) | 53.01 (52.90-53.14) | -5.160 | <0.001 |
| MDA | 1.50 (1.48-1.53) | 1.32 (1.28-1.34) | -5.162 | <0.001 |
a: Wilcoxon signed-rank test used to compare before- and after-treatment changes.
However, even after daily iron administration for eight weeks, SOD, CAT, and GPx levels continued to be significantly lower (p<0.001) in patients of IDA as compared to baseline levels in non-anaemic controls [Tables/Fig-6,7 and 8]. MDA levels in anaemic group at eight weeks remained significantly higher (p<0.001) than baseline levels in controls [Table/Fig-9].
Box Plot comparing pre- and post-iron therapy levels of SOD (units/mg protein).
Dark horizontal bar denotes Median, Box- interquartile range (25th–75th percentile), whiskers -Standard Deviation (SD), dark circles- outliers.
Box Plot comparing pre- and post- iron therapy levels of CAT (μmol H2O2 /min/ mg protein).
Dark horizontal bar denotes Median, Box- interquartile range (25th–75th percentile), whiskers -standard deviation (SD), dark circles- outliers, stars-extreme values.
Box Plot comparing pre- and post-iron therapy levels of GPx (nmol NADPH oxidized /min mg protein).
Dark horizontal bar denotes Median, Box- interquartile range (25th–75th percentile), whiskers -Standard Deviation (SD), dark circles- outliers, stars-extreme values.
Box Plot comparing pre- and post- iron therapy levels of MDA (nmols/ml of serum).
Box Plot comparing pre- and post- iron therapy levels of MDA (nmols/ml of serum).
Discussion
Being an important structural and functional component of various enzymes, viz. CAT, peroxidase, cytochrome oxidase, NADPH reductase and iron sulfur complex-dependent enzymes, iron is crucial for efficient anti-oxidant defences [19]. Furthermore, iron balance is also critical for maintaining normal erythropoesis [34]. In comparison to other cell types, the erythrocytes are equipped with a robust enzymatic machinery to handle and detoxify reactive oxygen and nitrogen species [16,35]. These enzymes, viz. SOD, CAT, Peroxidase, GPx, peroxiredoxin-2 and glutaredoxin-1 reverse thiol modifications on proteins, thus, safeguarding enzyme activities from oxidative inactivation [35]. However, the protective efficiency of erythrocytes against lipid peroxidation and carbonylation depends on the balance between oxidant species and the availability of anti-oxidant defenses [16,36].
The present study as well as several other studies [6–8,11,16,20,22,24,26], have reported a lowering of the anti-oxidant defenses in iron deficiency states [Table/Fig-10]. However, the causes of increased oxidative stress and decreased anti-oxidant defenses in IDA are still poorly understood. RBCs in IDA have increased susceptibility to haemolysis by inhibition of membrane sulfahydryl groups, increased membrane rigidity and decreased deformability. Moreover, oxidative insult may also induce immune recognition, resulting thereby in removal of RBCs from circulation [37]. A rise in reduced glutathione concentration along with increased enzymatic activity of both the Embden-Meyerhof and Pentose Phosphate Pathways (PPP) has been reported in children with IDA [29]. This increased energy and redox potential of iron deficient cells is believed to represent a compensatory mechanism secondary to defective synthesis of Hb and stroma. Contrary to this, some other researchers reported a reduction in PPP activity in IDA [8].
Studies on markers of oxidative stress and antioxidant status in children with iron deficiency anaemia.
| Study | Subjects | Agegroup | Parameters of OS or AO status |
|---|
| TOS | TAC | MDA | OSI | GSH | SOD | GSHPx | CAT | Others |
|---|
| El-Shimi et al.,2013 [26] | IDA=50 | < 2yrs. | - | IDA | IDA | - | - | - | - | - | - |
| C=50 |
| Altun et al.,2014 [25] | IDA=30 | 6 mths-15yrs. | - | - | NS | - | - | IDA | IDA | IDA | - |
| C=38 |
| Aycicek et al.,2014 [24] | IDA=65 | 1-16yrs. | IDA | IDA | - | IDA | - | - | - | - | IDA: -SH |
| C=28 |
| Akca et al.,2013 [23] | IDA=40 | 6 mths-5yrs. | IDA | NS | - | - | - | - | - | - | - |
| C=40 |
| Akarsu et al.,2013 [22] | IDA=20 | 6mths-12yrs. | - | IDA | - | - | - | - | - | - | - |
| C=20 |
| Bay et al.,2013 [21] | IDA=22 | 1-15yrs. | NS | NS | - | IDA | - | - | - | - | - |
| C=21 |
| Calik et al.,2013 [20] | IDA=19 | < 4yrs. | IDA | IDA | - | IDA | - | - | - | - | - |
| C=35 |
| Meral et al.,2000 [15] | IDA=19 | 2-16yrs. | - | - | - | IDA | - | NS | NS | - | IDA: CD |
| C=14 |
| Hafez et al.,1999 [13] | IDA=30 | 10 mths-10yrs. | - | - | - | - | - | IDA | - | - | - |
| C=10 |
| Sevgi et al.,1986 [11] | IDA=30 | rangeNA | - | - | - | - | - | - | IDA | - | - |
| C=25 |
| Tekin et al.,2001 [7] | IDA=15 | 1.5–15yrs. | - | - | - | - | - | NS | IDA | NS | - |
| C=10 |
CD: conjugated diene, SH: Serum total thiol, NS: not significant
IDA - Iron Deficiency Anaemia
In contrast to observations of the present study, some researchers observed neither an increase in lipid peroxidation nor any significant alteration in anti-oxidant enzyme activity in IDA [15]. Some others reported that, though there was no significant difference in MDA levels between IDA and controls, the levels of CAT, GPx and SOD were significantly higher in IDA [25]. An earlier work reported similar SOD and CAT activities in both IDA and control groups, but significantly lower GPx activity in the IDA group [7]. In another study, levels of glutathione reductase were observed to be normal in IDA, whereas SOD activity was found to be increased [12]. A study done later reported significantly higher CAT and SOD levels, comparable GPx activity, and significantly increased MDA production in patients with IDA as compared to controls [10]. On the contrary, some workers reported that whereas SOD activity was significantly lower in adults with IDA, the GPx activities were similar to controls [16]. Increased lipid peroxidation despite high activity of glutathione reductase and glucose 6-phosphate dehydrogenase has also been reported in patients of IDA [38]. A few of the studies conducted on children estimated Total Oxidative Stress (TOS), Total Antioxidant Capacity (TAC) and /or Oxidative Stress Index (OSI) in IDA and control group [20–24,26]. While most of these observed lower TAC in IDA patients [20,22,24,26], two studies additionally reported higher TOS and OSI in IDA as compared to controls [20,24]. In one of these studies, TOS was observed to be significantly higher in IDA group, while TAC was observed to be similar to control group [23]. Contrary to the findings of all these studies [20–24,26], some scholars observed no significant alteration in either TOS or TAC but an increase in OSI in cases with IDA [21].
In the present study, levels of Hb and markers of iron status (serum iron, transferrin saturation and serum ferritin) were observed to have a highly significant, positive correlation (p<0.001) with levels of markers of anti-oxidant status (SOD, CAT, and GPx). TIBC, on the other hand, had a highly significant (p<0.001) but negative correlation with these markers. Likewise, in a previous report, serum iron and ferritin levels were found to have a strong positive correlation with CAT, GPx and SOD activity [39]. Another study on children reported, an inverse correlation between Hb and SOD values, but no correlation with other anti-oxidant enzyme activities [25]. Some researchers, on the other hand, observed no correlation between either serum iron or ferritin and anti-oxidant capacity [16,40]. Levels of Hb and all markers of iron status (except TIBC) were observed to have a highly significant negative correlation (p<0.001) with MDA in the present study. These findings are in agreement with results of a recent Indian study on iron deficient pregnant anaemic women, where median values of enzymatic and non-enzymatic anti-oxidants (except oxidized glutathione) were observed to decrease linearly with severity of anaemia, whereas all oxidative parameters, viz. lipid peroxidases, protein carbonyls and conjugated dienes increased linearly with severity of anaemia [39]. On the other hand, in a very recent research work, MDA levels were reported to have a strong, statistically significant positive correlation with serum iron but no significant correlation with either ferritin or Hb levels in iron deficient pregnant women [40]. Moreover, a strong reciprocal correlation was also observed between Hb levels and TAC in these patients [40]. Some other recent studies done on children, however, reported no correlation between Hb, measures of iron status and indicators of oxidative stress or antioxidant status in IDA [13,17,23,24].
Conflicting reports are available from various animal and human studies evaluating the effect of iron supplementation on markers of oxidative stress and anti-oxidant status in patient with IDA [4,11,16–19,22–26–29,41–49] [Table/Fig-11]. As early as 1968, researchers had reported a significant lowering of elevated reduced glutathione levels after iron therapy in children with IDA [29]. Even though increased oxidative stress along with weakening of anti-oxidant defences is known to occur in iron deficient states [20,24,26], iron, being a redox-active metal, itself has the ability to participate in the Fenton reaction and generate reactive oxygen species in certain situations [41–43]. Even pharmacologic doses/moderate iron supplementation have been reported to increase the amount of free iron available for free radical generation and lipid peroxidation [25,28,44,45]. In a study on women with low iron stores, a more than 40% increase from baseline was observed in plasma MDA and Breath Ethane Exhalation Rates (BEER) after six weeks of daily iron supplementation in the usual recommended doses of 98 mg per day [18]. A study on Turkish children reported a rise in MDA levels along with a concomitant fall in the previously raised levels of SOD, CAT and GPx following three months of oral iron therapy (4mg/kg/day) [25]. These findings are contrary to those of the present study as well as some other studies where a highly significant (p<0.001) rise in levels of SOD, CAT and GPx, accompanied by a highly significant (p<0.001) decline in MDA levels was observed following 6-8 weeks long iron treatment [4,46]. Similarly, a prior study from Turkey had reported a significant rise in GPx levels of eight children with IDA following oral iron supplementation for three months [11]. A significant rise in SOD activity following oral iron therapy for 6 months has been reported previously in adults [16]. Some workers failed to find any significant change in levels of SOD, GPx, urine 8-isoprostane or basal and Cu-stimulated-oxidized LDL at either 3 or 6 months of iron treatment. They, however, observed a significant decline in MDA and CAT levels at six months in children receiving ferric and ferrous salt, respectively [17]. Likewise, a previous study on Indian children reported a declining trend in lipid peroxides and lipid hydroperoxides following oral supplementation with 6mg/kg/day of elemental iron [19]. A recent Turkish study on children with IDA reported that whereas TOS decreased significantly after treatment with iron, TAC remained unaltered [23]. Some investigators, however, reported a rise in TAC following oral iron [22,26]. In the present study, the mean levels of antioxidant enzymes in patients with IDA continued to be significantly lower (p<0.001), while those of MDA remained significantly higher (p<0.001) in comparison to controls even after eight weeks of therapy. Our results are in conformity with findings of some others who found post-treatment antioxidant activity in patients with IDA to be still lower, and oxidative stress still higher than in controls [22,24,26]. These observations differ from those of some others who reported oxidant activity, anti-oxidant capacity and catalase activity to reach control values post-supplementation [46]. Some other workers in this field, on the contrary, failed to find any relationship between iron intake or iron status and lipid peroxidation in humans [47–49]. However, considerable variation existed in the methodology adopted in all these studies vis-à-vis the dosage, route, type of iron preparation, duration of iron supplementation, and/or the markers of oxidation and antioxidant status studied.
Studies exploring effect of oral iron therapy on markers of oxidative stress and antioxidant status in children with Iron Deficiency Anaemia (IDA).
| Study | Subjects | Age group | Parameters of OS or AO status |
|---|
| TOS | TAC | MDA | OSI | GSH | SOD | GSHPx | CAT | Others |
|---|
| El-Shimi et al.,2013 [26] | IDA=50 | < 2yrs. | - | IDAPost TIDA< C | IDAPost TIDA>C | - | - | - | - | - | - |
| C=50 |
| Altun et al.,2014 [25] | IDA=30 | 6 mths-15yrs. | - | - | Post TIDA>C | - | - | Post T IDA=C | Post T IDA=C | Post T IDA=C | - |
| C=38 |
| Aycicek et al.,2014 [24] | Fe2+ grp= 33 | 1-16yrs. | Fe2+grp:at 1mth Post TIDA>CFe3+:grp:NS | NS | - | Fe2+grp:OSI Post TIDA>C | - | - | - | - | -SH:NS |
| Fe3+ grp=32 |
| Akca et al.,2013 [23] | IDA=40 | 6 mths-5yrs. | Post TIDA=C | NSPost TIDA=C | - | - | - | - | - | - | - |
| C=40 |
| Akarsu et al.,2013 [22] | IDA=60 | 6 mths-12yrs. | | IDA< C7d,6wks, 13wks | - | OSINS | - | - | - | - | - |
| C=20 |
| Krishnamoorthy et al.,2010 [19] | IDA=11 | range NA | - | - | - | OSI | - | - | - | - | - |
| IDA=72 |
| Kavaklı et al.,2004 [17] | Fe2+/ =39 | 6 mths-15yrs. | - | - | 3mths:NS6mths:Fe 3+:Fe2+:NS | - | - | NS at 3 & 6mths | NS at 3 & 6mths | 3mths:NS6mths:Fe2+:Fe 3+: NS | U- 8- isoprostane, Basal & Cu- ox- LDL:NS at 3 & 6mths |
| Fe3+ =33 |
OS: oxidative sress, AO: antioxidant, C: controls, NS: not significant, Post T: Post therapy, U- 8-isoprostane: urine 8-isoprostane, Basal and Cu- ox- LDL: basal and Cu-stimulated-oxidized LDL, -SH: Serum total thiol; IDA - Iron Deficiency Anaemia; TOS - Total Oxidative Stress; TAC - Total Anti-oxidant Capacity; MDA - Malondialdehyde; OSI - Oxidative Stress Index, SOD - Superioxide Dismutase; CAT- Catalase.
Limitation
Since, the sample size for this sub-study included anaemic participants of a main study (RCT exploring the effect of 2 different dose iron regimens in children with IDA), it is likely that the sub-study might not have had the desirable power to detect differences in levels of markers of oxidative stress and antioxidant status. In addition, certain other parameters such as TOS, TAC, OSI, TBARS, CD etc., which had been studied by some other researchers could not be estimated by us. The study was also somewhat limited by its short duration of iron supplementation and follow-up.
Conclusion
The findings of the present study suggest that oxidative stress is increased, whereas anti-oxidant defenses are lowered in children with IDA; oral iron therapy can, effectively, mitigate all these changes. However, an 8 week period is probably not sufficiently long enough to completely reverse the imbalance between anti-oxidant status and oxidative stress occurring in these cases.
Further large scale, preferably, long term studies, using different iron regimens need to be conducted to determine the adequate duration of iron therapy required to reverse all these changes in this important and fairly common nutritional disorder.
*: Data expressed as mean±standard deviation (SD); Independent samples t-test used for comparisons.
#: Data expressed as median with Interquartile Range (IQR) given in parenthesis; Mann–Whitney test used for comparisons.
IQR: Interquartile range
CD: conjugated diene, SH: Serum total thiol, NS: not significant
IDA - Iron Deficiency Anaemia
OS: oxidative sress, AO: antioxidant, C: controls, NS: not significant, Post T: Post therapy, U- 8-isoprostane: urine 8-isoprostane, Basal and Cu- ox- LDL: basal and Cu-stimulated-oxidized LDL, -SH: Serum total thiol; IDA - Iron Deficiency Anaemia; TOS - Total Oxidative Stress; TAC - Total Anti-oxidant Capacity; MDA - Malondialdehyde; OSI - Oxidative Stress Index, SOD - Superioxide Dismutase; CAT- Catalase.