In patients with advanced carcinomas of the head and neck, locoregional control poses a major therapeutic challenge. For unresectable tumours, the treatment has been conventionally given fractionated Radiation Therapy (RT) up to total doses of 60–75 Gy administered in 6–8 weeks and resulted in 2-year survival rate of less than 30%. Attempts to improve this poor outcome have been made by combining conventional RT with concurrent Chemotherapy (CT) or introducing different alterations of radiation fractionation using hyperfractionated and/or accelerated treatment schedules [1–5]. The concurrent administration of CT and RT has shown to be associated with better tumour response as compared to RT alone [6].
Accelerated repopulation of tumour clonogens during the conventional fractionated RT is an important determinant of local control and possible cause of treatment failure in head and neck carcinoma, especially if the overall treatment time is prolonged. These observations led to the development of accelerated fractionation schedule, whereby radiotherapy is administered in a shorter overall time for improving tumour control. Hyperfractionation has shown to be an advantage in clinical radiotherapy. In view of these observations, the present study was undertaken to find out the comparative efficacy and adverse reactions of conventional concurrent Chemo-Radiotherapy (CRT) and Accelerated Hyperfractionation (AHF) in patients of advanced stages of carcinoma of head and neck.
Materials and Methods
This is a prospective randomized trial. The study flow is shown in [Table/Fig-1]. The study was conducted from February 2012 to July 2013 (total duration -one year and six months). A total of 54 patients of carcinoma head and neck were screened from RT out-patient department of the institute between February 2012 to February 2013. Out of these, 30 patients satisfied the inclusion criteria. The study included patients of unresectable stage IV A and IV B (AJCC cancer staging 2010) of squamous cell carcinoma of head and neck in the age group of 30-65 years of both gender. They also had Eastern Co-operative Oncology Group (ECOG) performance status of grade 0, 1 and 2 and normal liver, renal functions, complete blood count, ECG and X- ray chest. The excluding criteria were patients with past history of cancer, already received CT or RT before registration, having ECOG Performance Status of Grade > 2, insufficient data and any serious medical condition that could jeopardize the safety of the patient and/or the efficacy assessment of the study. Thereafter, they were randomized into two treatment arms- Arm A and Arm B by computer generated random table number. Sample size was calculated according to expected patient load in the department. Minimum of 15 patients were planned to be enrolled in each arm. The protocol of the study was approved by Institute Ethics committee and informed consent was taken from patients/authorized representative of patient.
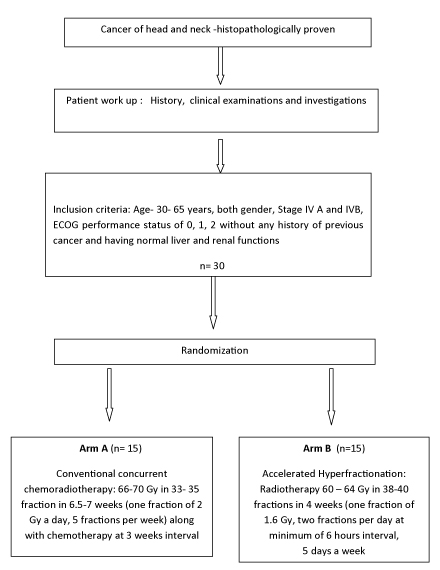
Arm A consisted of conventional RT delivering total dose of 66 -70 Gy in 33-35 fractions in 6.5 to 7 weeks (2 Gy/fraction, 5 days in a week) to gross primary and nodal disease along with concurrent CT. Using shrinking field technique spinal cord was spared after 44Gy/22 fraction/ 4½ weeks and then remaining dose was delivered with the same fractionation schedule.
Regarding CT, the protocol was to give 3 cycles (along with concurrent RT) with an interval of 3 weeks between the cycles. Before each cycle, complete blood count, liver function and renal function tests were done and CT was administered only when reports were normal. The schedule consisted of intravenous Paclitaxel (175mg/ m2) on day 1 and Cisplatin (100mg/ m2) in 3 divided doses from day 1 to day 3 of each cycle. Patients with creatinine clearance of less than 50ml/1.73m2/min were given Carboplatin 5 times AUC on Day 1 of each cycle in place of Cisplatin. First dose of first CT cycle was given on day 1 of start of RT.
Arm B consisted of AHF schedule delivering total dose of 60 - 64 Gy in 38-40 fractions in 4 weeks (1.6 Gy/#, 2# per day at minimum of 6 hours interval, 5 days a week) to gross primary and nodal disease. Using shrinking field technique spinal cord was spared after 48Gy/30 fraction/3 weeks and then remaining dose was delivered with the same fractionation schedule.
For delivering RT the patients were made to lie in the most comfortable anatomical position (supine position with head and neck in neutral position, resting on the head rest) on the treatment table to decrease undue mobility of the patients and enable reproducibility of the planned setup. Different sizes of head rest were used to match the degree of neck extension needed. For immobilization, thermoplastic casts were used. Fields marking was done according to the clinical assessment of target volume. Cobalt-60 teletherapy machine was used for irradiation therapy. The treatment protocol was followed for both the arms as mentioned above.
Patients were evaluated weekly during treatment. After treatment completion follow-up was done monthly. Their complaints and acute reactions were noted. Radiation Therapy Oncology Group (RTOG) Acute Radiation Morbidity Criteria and RTOG/EORTC (European Organisation for Research and Treatment of Cancer) Late Radiation Morbidity Criteria were used for toxicity assessment. Appropriate treatment was prescribed during the follow-up when needed. Response to treatment was assessed after 3 months of treatment completion in both the arms as part of treatment protocol. Response evaluation was done by clinical assessment and imaging. Biopsy or cytology was done when required. RECIST (Response Evaluation Criteria In Solid Tumours) version 1.1 was used for response assessment.
Data collection and analysis was done between March 2013- July 2013. Statistical analysis was done using SPSS 17.0 software version. Parameters were analysed by comparing results of the two treatment arms using the Fisher’s-Exact Probability test.
Results
The basic demographic data of two groups are presented in [Table/Fig-2]. The patient and tumour related characteristics were comparable in both the arms. The most common site was oral cavity as site of origin and had T4a disease status in both the arms, while N2b status was most common in Arm A while N2c was in arm B.
Patient’s demographic data.
| Characteristics | CombinedChemotherapy andRadiotherapyArm A (n=15) | AcceleratedHyperfractionationArm B (n=15) |
|---|
| Age (Median) | 53 yrs | 53 yrs |
| Sex - Male | 12(80%) | 12(80%) |
| Female | 3(20%) | 3(20%) |
| Religion - Hindu | 15(100%) | 12(80%) |
| Muslim | 0(0 %) | 3(20%) |
| Inhabitance - Rural | 10(67%) | 10(67%) |
| Urban | 5(33%) | 5(33%) |
| Primary site-Oral cavity | 9(60%) | 12(80%) |
| Oropharynx | 4(27%) | 3(20%) |
| Hypopharynx | 1(7%) | 0(0%) |
| Larynx | 1(7%) | 0(0%) |
| Grade- 1 | 9(60%) | 8(53%) |
| 2 | 2(13%) | 3(20%) |
| 3 | 2(13%) | 2(13%) |
| Unknown | 2(13%) | 2(13%) |
| T status – T 2 | 1(7%) | 0(0%) |
| T3 | 1(7%) | 0(o%) |
| T4a | 12(80%) | 15(100%) |
| T4b | 1(7%) | 0(0%) |
| N status – N0 | 3(20%) | 1(7%) |
| N1 | 2(13%) | 4(27%) |
| N2a | O(0%) | 2(13%) |
| N2b | 7(47%) | 3(20%) |
| N2c | 3(20%) | 5(33%) |
| Stage – IV A | 14(93%) | 15(100%) |
| IV B | 1(7%) | 0(0%) |
n – number of cases.
Treatment compliance in Arm A
Two patients in Arm A did not come for treatment. Out of remaining 13 patients, 12 (92%) had completed the treatment (one patient expired during the treatment due to myelosuppression and associated infection). The mean duration of treatment completion was nine weeks (7.5-12.1 weeks) and the mean prolongation of treatment was two weeks (0.5-5.1 weeks).
Out of these 12 patients, 6(50%) patients had received three cycles of concurrent CT. Other Six patients could not be given concurrent 3rd cycle CT due to toxicity. Patient who expired during the treatment had received only two cycles of CT.
All 12(92%) had received RT dose of 70Gy|35# and the one patient who could not complete treatment and had received 44 Gy| 22#. In all cases, field shrinkage was done at the dose of 44Gy|22# and spinal cord was kept out of the field and with provision of one to two more shrinking of the field, total dose was delivered. Along with RT, CT was given on d1, d22 and d43 as feasible depending on the patient’s tolerance.
Treatment compliance in Arm B
Out of 15 patients, 13 (87%) had completed the treatment (one expired during treatment due to cardiac event and another one left the treatment in between). Eight patients (53%) had received RT dose of 60.8Gy|30#, 5 (33%) patients received 64 Gy|40# and the 2(13%) patients who did not complete the treatment had received 25.6Gy|16#. In all the 13 patients, total dose was given according to the patient’s toxicity profile. Field shrinkage was done at the dose of 48Gy|30# and spinal cord was kept out of the field and with provision of one to two more shrinking of the field, total dose was delivered. The mean duration of treatment completion was 6 weeks (4-9.2 weeks). The mean prolongation of treatment was 2 weeks (0-5.2 weeks).
Follow-up
Of 30 patients, 28 (93%) patients came for the treatment.
Toxicity during Treatment
Out of 28 patients who turned up for treatment, 18 (64%) cases developed grade three mucositis (eight in arm A and ten in arm B). The grade three reactions were observed in arm A after completion of four weeks and in arm B after completion of three weeks of treatment. Five (38%) patients in arm A and four (26%) in arm B developed grade 2, while one patient (of two who did not complete the treatment) in arm B developed grade 1 mucosal reactions. The acute mucosal reactions in both the arms took mean period of 2.5 months to heal.
Grade three skin reactions (desquamation of skin) occurred in 26 (93%) cases (13 in each arm). Two patients in arm B who did not complete the treatment, showed grade two skin reactions (desquamation of skin). All the patients who showed grade three skin reactions, showed after completion of four weeks of treatment in arm A and after three weeks in arm B.
Out of 28 patients, eight (62%) in arm A and 1(7%) in arm B developed myelosuppression. Four cases developed infections due to myelosuppression and all were in arm A (2 septicaemia, 1 respiratory tract and one gastrointestinal tract infection).
Toxicities were managed by appropriate symptomatic and supportive measures (antibiotics, antifungal agents, nutritional support, GCSF support, analgesics, strict oral hygiene and others). Seven (54%) cases in arm A and five (33%) in arm B required hospitalization for toxicity management. Seventy seven percent (n=10) patients in arm A and 73% (n=11) in arm B required Ryle’s tube insertion because of tumor bulk and severe acute mucosal reactions.
Follow-up Details
Out of 28 patients who came for the treatment, at three months, 19 (67.8%) turned up for follow-up (nine (47.36%) in Arm A and 10 (52.6%) in Arm B).
In Arm A, three patients expired despite of best possible specific and supportive care. One patient expired during the treatment, one after two weeks of treatment completion due to myelosuppression and associated infection. One patient expired after one month of treatment completion at his native place due to unknown reason. One patient lost to follow-up after one month of treatment completion.
In Arm B, three patients lost to follow-up after one month of treatment completion. One patient expired during the treatment due to cardiac event and one patient left the treatment in between.
After six months, eight (29%) patients turned up for follow-up (three (23%) in Arm A or five (33%) in Arm B).
In Arm A, four patients could be followed only up to three months and one patient lost to follow-up after three months. One patient was sent to surgery for residual disease. In Arm B, three cases could be followed up to three months and two cases had residual disease (one patient sent to surgery and another patient refused any further treatment).
Biological Equivalent dose (BED)
In the present study, the radiobiological parameters BED10 (planned), BED10 (time corrected) and BED3 were calculated using Linear Quadratic Formulation. The mean BED10 (planned), BED10 (time corrected) & BED3 in arm A were 84, 61 and 117, respectively while in Arm B was 72, 63 and 95, respectively. In this present study, the BED 10 (time corrected) values were equated for both the treatment regimes.
Response
At 3 months follow-up, total of 19 patients came for evaluation. Out of these, 16 were in complete response. Out of 13 patients in arm A and 15 in arm B who came for treatment, eight (62%) in arm A and eight (53%) in arm B had complete response, however, difference was not statistically significant) and two (13%) had residual disease in arm B (one was sent for surgery while another patients refused any further treatment). One patient in arm A had residual disease and was sent for surgery. Subsequently at six months, out of eight patients who turned up for follow-up, seven had complete response (three in arm A and four in arm B) and one patient had recurrence of disease (sent for surgery). Thereafter, only one patient could be followed at nine and 12 months follow-up who had complete response.
Overall survival
The mean overall survival of total number of patients selected for the study was 5.96 months. This was 6.42 months and 5.76 months in Arm A and B, respectively.
Discussion
Various studies have shown median age of carcinoma head and neck patients ranging between 52-55 years [7,8]. The present study also found similar result with median age of 53 years. Male preponderance (83%) was found in the present study and this is in accordance with the observation by Chauhan et al., [8]. The majority of the patients were Hindus and more than half of patients were from rural background which was just reflection of population belonging to different religion and background.
About 2/3rd of the cases had ECOG performance status of 1 whereas 33% had 0 status. Somani et al., found that 90% of the cases had ECOG status of 1 [7]. We had about 1/3rd patients with ‘0’ status, may be because they were referred little earlier with a better general condition.
The most common site was oral cavity (70%), followed by oropharynx (23%), Hypopharynx (3%) and larynx (3%) in the present study. Yogi and Singh found similar finding of commonest site being oral cavity [9]. In contrast, Somani et al., found pharynx being the most common site with percentage incidence of larynx similar to as in our study [7].
In the present study, all of the patients had squamous cell carcinoma; 53% cases had well differentiated (Grade I), 17% had moderately differentiated (Grade II) and poorly differentiated (Grade III) each and in 13% cases the differentiation was not known. Denis et al., in their study found that 50% cases had grade I, 30% had grade II and 10% each grade 3 and unknown grade which is consistent with our study [10]. Maximum number of patients had T4a and N2 status, which was similar to study by Bourhis et al., which showed that more than 2/3rd patients belonged to T4 lesion and 50% had N2 disease [11].
There was delay in treatment completion in both the arms due to toxicity and treatment interruption by the patient. The treatment time result (mean = six weeks) in arm B is supported by study conducted by Jackson et al., [12]. However, the results of GORTEC trial showed mean overall time of 22 days in accelerated arm [11]. After 3 months, the response to treatment was better in CRT arm as compared to AHF but it was not significant. Similarly, there was little difference in response rate at six months also.
Most of the patients had grade three mucosal and skin reactions in both the arms. In arm A, it was because of concurrent CT while in arm B, it was due to accelerated treatment. Accelerated regimens have been shown to increase treatment associated acute morbidity as reported by previous authors [11,12], which showed majority of patients developed grade 3 reactions. The skin and mucosal reaction hold significance in the fact that along with myelosuppression they had influenced the treatment schedule and treatment duration. In arm A, 46% patients could not be given the 3rd cycle CT and in arm B only in 33%, 64 Gy could be given due to toxicities. Three patients in arm A and one patient in arm B expired. In arm A, it seemed besides toxicities, elderly age, relatively low ECOG status and advanced stage of the disease were the causative factors, while in arm B, the death was related to cardiac event.
Limitation
Limitations of the present study are small sample size and shorter duration of follow-up.
Conclusion
Thus, it is obvious that being a referral care centre, we received cases of carcinoma head and neck in advanced stage of disease. The treatment outcome in both the regimen establishes the fact that AHF treatment is able to match the efficacy of chemo- radiotherapy treatment with lesser toxicity in locally advanced unresectable squamous cell head and neck cancer. So the present study concludes that in such cases AHF should be preferred over CRT. However study on larger sample size and with longer duration of follow-up is required to find out safety and efficacy of the individual treatment regimen.
n – number of cases.