Diabetes Mellitus (DM) is a metabolic disorder; alarmingly increase in the incidence observed globally. DM considered as a major threat to human health in developing and developed countries due to its complications. In spite of control of DM, complications may develop over due course of disease. Neuropathy is the most common chronic complication of DM affecting more than 50% of the patients [1]. The duration of disease and level of hyperglycaemia are important determinants of microvascular complications of diabetes [2]. Pain due to diabetic neuropathy affects most often lower extremities above the knees and upper extremities [3]. Abnormal excitement of immaturely regenerated nerve fibers causes spontaneous pain, numbness and paresthesia [4]. Pain occurs even after the exposure to mild pain stimuli i.e., hyperalgesia and allodynia. Diabetic neuropathy shows significant impact on Quality Of Life (QOL) and costs of management [5]. Apart from tight glycaemic control, no other evidence-based treatments are known to prevent neuropathy [6].
The exact pathology of diabetic neuropathy is not known, but it involves oxidative stress, advanced glycation end products, polyol pathway flux activation contribute to microvascular disease and nerve dysfunction [7]. Current treatment based on the use of desipramine, amitriptyline, capsaicin, tramadol, gabapentin, bupropion, and venlafaxine etc., [8]. But these drugs provides only symptomatic relief and has no effect on Blood Sugar Level (BSL) and progression of disease.
Materials and Methods
Wistar rats of either sex having average weight of 150-200g were used for the study. Housing was done in accordance with Committee for the Purpose of Control and Supervision of Experiments on Animals (CPCSEA) guidelines in standard cages. The animals were given food and water ad libitum and were exposed to12 hours light and dark cycle. Study started after obtaining the approval (IAEC:BVDUMC/88/2014-15) from Institutional Animal Ethics Committee of the Bharati Vidyapeeth University Medical College, Pune (CPCSEA - 258) Maharashtra, India.
Plant material
Dried Curcuma longa rhizomes and fresh fruits of Emblica officinalis were collected from the local market, Pune (Maharashtra). The plant material was authenticated by Ayurvedic College of Bharati Vidyapeeth, Pune, India.
Preparation of Nishamalaki formulation
Amla extract was added to the Curcuma powder in proportion of 6:1. This mixture was triturated till it become fine paste. The mixture was shed dried. Completely dried product was again powdered and stored in clean dry glass container. The final product NA was given to rats in the dose of 0.9g/kg with honey. Doses of NA (0.9gm/kg, p.o.) were selected on the basis of previous literature [14].
Drugs and Chemicals
Streptozotocin (STZ) was purchased from Sigma Aldrich, obtained from Anand agencies, Pune. Chemicals of analytical grade were purchased from local suppliers of Pune.
Induction of Diabetes
STZ was dissolved in ice cold citrate buffer (pH 4.4). Drug solution was prepared fresh. Group I (Control) vehicle consists of 6 rats. Type 2 Diabetes induced in 36 wistar rats with STZ (35mg/kg) intra-peritoneally single injection followed by High Fat High Fructose Diet (HFHF). HFHF consists of Vanaspati Ghee and Coconut oil inproportion of 2:3 and 10% fructose for drinking [15]. After confirmation of development of diabetes; rats were divided into six groups of (n=6).
Groups:
» Gr I: Control –Saline Treated
» Gr II: DM control- Saline Treated
» Gr III: Nishamalaki- Low dose- 0.9gm/kg
» Gr IV: Nishamalaki- High dose- 1.8gm/kg
» Gr V: Glibenclamide- 5mg/kg
» Gr VI: Pioglitazone- 2.7mg/kg
» Gr VII: Epalrestat- 4mg/kg
Animals received drug treatment for next 12 weeks as per the groups. Monitoring of BSL was done on 15 days and lipid profile at the end of the study.
Neuropathy Assessed With
Sensory component: Reaction to thermal stimulation- hyperalgesia is an early symptom of neuropathic pain and diabetic neuropathy pain in experimental animals. Hot-plate method [16].
Animals were individually placed on Eddy’s hot-plate apparatus (LE 7406) that contained a plate with the diameter of 25cm and a plexiglas wall with height of 20cm. The temperature adjusted to 52 ± 1°C. To calculate latency time from putting the animal on the hot-plate to the first sign of paw licking or jump response to avoid the heat was taken as an index of the pain threshold. The cut-off time of 10 seconds was given in order to avoid damage to the paw to all the animals [17].
Tail-immersion (hot water) test [
18]
The lower 5cm portion of the tail is marked. Tail of rat was immersed in a hot water bath with temperature 52.5 ± 0.5 °C. Within a few seconds the rat reacts by withdrawing the tail (flicking response) or signs of struggle were observed (cut-off 12 seconds). Shortening of the tail withdrawal time indicates hyperalgesia. After each determination the tail is carefully dried. Rat was gently placed in the cage.
Assessment of Cold allodynia [
19]
Cold allodynia was assessed by measuring tail withdrawal latency. Ice cold water (4±10°C) was taken in beaker. The tail of rat was submerged gently in water and the withdrawal time was measured for diabetic neuropathy. A cut-off 20 seconds was maintained throughout the experiment.
Motor Component
Assessed with Walking function test [
20]
Walking test was performed on a rod of 6cm diameter and 1 m long for assessment of motor component. Rod was placed horizontally 40cm above a ground. The rod was graduated in order to allow the measurement of the distance covered by the animals. Three trials per session were given. Each trial was of 60 seconds maximum, each rat was placed at one end of the rod and the time taken cover the distance of 1m was recorded. If the animal falls down or is unable to walk the 1m distance, 60 s were credited. For each animal, the mean duration of the three trials was calculated and retained as the characteristic value.
End of the experiment- All the rats were sacrificed and sciatic nerves were carefully removed for antioxidant activity and histopathological study.
Estimation antioxidant parameters
Nerves tissues were incubated separately in culture medium Tc 199 for 48 hours. Tissue homogenized in 0.1M sodium phosphate Buffer in homogenizer. Homogenate was centrifuged for 30 min at 4°c. Supernatant collected and stored at 20°c. Supernatant of homogenates was used to estimate lipid peroxidation (MDA content), Sodium Dismutase (SOD) and catalase levels.
Histopathology of sciatic nerve
The nerve tissues were washed with saline and fixed in 10% formalin. Slides were prepared by embedding the nerve in paraffin and cut into 4μm thickness sections. Slide staining was done with Haematoxylin and Eosin (H&E). Nerve sections were analysed under light microscope for axonal degeneration and vascular defects
Statistical Analysis
Results were expressed as mean±SEM. The data were analysed by one-way Analysis of Variance (ANOVA) followed by Tukey’s multiple comparison tests. p<0.05 was considered statistically significant.
Results
Reaction to thermal stimulation-
Diabetic rats exhibited significant (p<0.001) hyperalgesia compared with control rats in both the tail-immersion and hot-plate method. Drug treatment showed improvement in all drug treated groups. NA administration to diabetic rats produced a dose dependent increase in pain threshold as compared to untreated diabetic rats in both tail-immersion and hot-plate assays [Table/Fig-1]. Epalrestat (p<0.001) was more effective in tail immersion than hot plate method.
Reaction to thermal stimulation.
| Groups | Hot plate(Reactiontime in sec) | Hot waterimmersion test(Reactiontime in sec) | Cold waterimmersion(allodynia)test (Reactiontime in sec) |
|---|
| Normal Control | 8.5±0.76 | 11.16±0.47 | 12.66±0.91 |
| Diabetic Control | 2.3±0.33$$$ | 3.6±0.33$$$ | 5.00±0.51$$$ |
| NA low dose | 6.1±0.90* | 8.1±0.94**$ | 10.66±1.30** |
| NA High dose | 7.1±0.60** | 9.5±0.99*** | 12.33±0.80*** |
| Glibenclamide | 6.5±0.76* | 8.0±0.68*$ | 10.33±0.42** |
| Pioglitazone | 6.0±0.73* | 8.1±0 .47**$ | 10.83±0.60** |
| Epalrestat | 6.6±0.42* | 8.8±0.54*** | 10.66±0.66** |
Data expressed as Mean± SEM, $$$p<0.001 compared with control and * p<0.05, ** p<0.01, *** p<0.001 in comparison with diabetic control
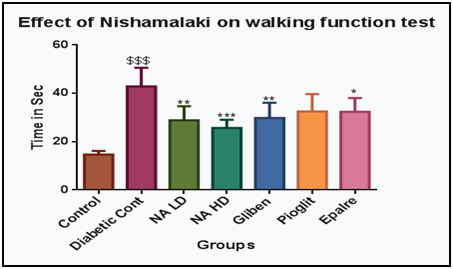
Walking function
Significant increase in time taken to travel 1m distance on rod in DM control (p<0.001) was observed. There was decrease time seen in all treated rats compared to DM group rats [Table/Fig-2]. Highest efficacy was seen in NA high dose (p<0.001). Epalerstat (p<0.05), low dose NA (p<0.01) and Glibenclamide (p<0.01) was similar in walking efficacy. Non significant decrease was seen in Pioglitazone group [Table/Fig-3].

Effect of nishamalaki on walking function test.
Data expressed as Mean± SEM, $$$p<0.001 compared with control and * p<0.05, ** p<0.01, *** p<0.001 in comparison with diabetic control.
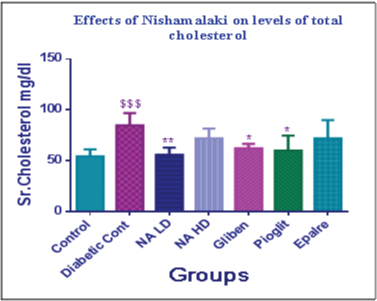
Lipid Profile
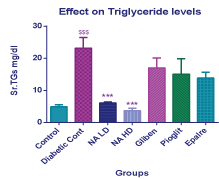
Effect of NA on serum cholesterol and serum triglycerides was evaluated. Serum cholesterol levels in diabetic control group shows significant increase (p< 0.001) than normal control [Table/Fig-4]. NA in 0.9gm/kg dose showed significant decrease (p< 0.01) in cholesterol levels. Glibenclamide and Pilglitazone showed less effect then NA (p< 0.05). Epalrestat was very less effective in reducing cholesterol. In case of Serum triglycerides similar pattern was observed in diabetic control group. There was significant reduction in the levels with both the doses of NA (p<0.001) was seen [Table/Fig-5]. Favourable changes in lipid profile were observed with Nishamalaki.
Effect of nishamalaki on levels of total cholesterol.
Data expressed as Mean± SEM, $$$p<0.001 compared with control and * p<0.05, ** p<0.01, in comparison with diabetic control.
Effect on triglyceride levels.
Data expressed as Mean± SEM, $$$p<0.001 compared with control and *** p<0.001 in comparison with Diabetic control.
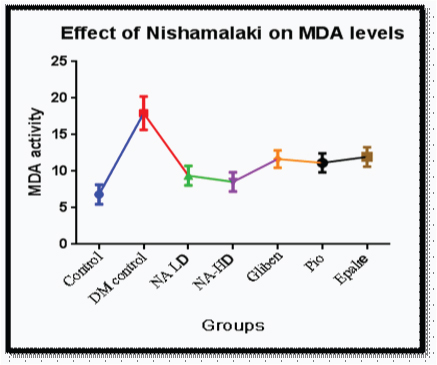
Sciatic nerve antioxidant activity
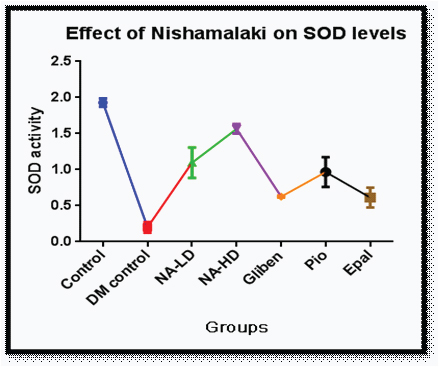
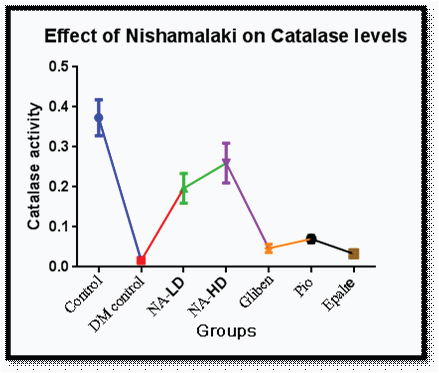
Levels of Malondialdehyde (MDA) were increased significantly (p<0.001) in diabetic control group compared to control indicative of oxidative stress [Table/Fig-6]. Decrease in the levels of MDA was seen with NA- HD. There was increase in levels SOD (p<0.001) [Table/Fig-7] and catalase (p<0.001) [Table/Fig-8] in NA group.
Effect of nishamalaki on MDA levels.
Effect of nishamalaki on SOD levels.
Effect of nishamalaki on Catalase levels.
Sciatic nerve histology
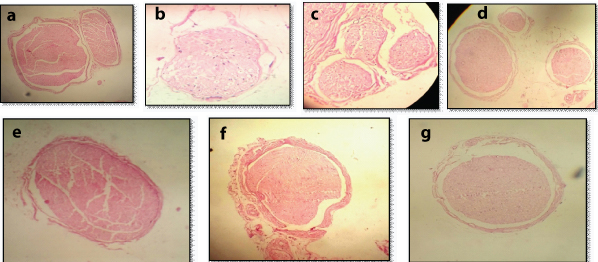
The diameter of nerve fibres in cross-section was markedly reduced suggesting the shrinkage of nerve fibres and marked axon atrophy and myelin vacuolization was evident in the cross-sectional view of the sciatic nerve in diabetic control group [Table/Fig-9a-g]. Normal arrangement of fibres and density of myelinated fibres was well maintained in NA group. Few areas of partial loss of fibres were evident in Glibenclamide and Epalrestat treated groups.
Histopathological Findings.
(a)–Control Normal -Fibers are normal and completely arranged. Density of myelinated fiber is well maintained.
(b)– DM Control - Loss of both large and small myelinated fibers.
(c)–Nishamalaki low dose-Partial loss of myelination large and smallfibers.
(d)–Nishamalaki high dose- Myelinated fibre density well preserved and are compactly arranged. Some regenerating clusters are seen.
(e)–Glibenclamide - The fibers are compactly arranged and partial loss of myelinated fibers in some areas.
(f)–Pioglitazone-The fibers are compactly arranged and myelinated fibers is well preserved.
(g)–Epalrestat-Partial loss of myelinated fibers in some areas.
Discussion
Diabetic polyneuropathy represents a diffuse symmetric and length-dependent injury to peripheral nerves [21]. It affects Quality of life, increases morbidity and cost treatment [22]. In DN- structural and functional aberration of peripheral neurons, spinal glial cells and nerve [23]. The hyperalgesic response on the hot-plate is considered to result from a combination of central and peripheral mechanisms [24]. Allodynia is hallmark of neuropathic pain and diabetic neuropathy pain in experimental animals [25].
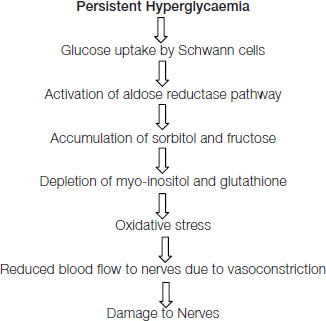
Various mechanisms for toxicity on peripheral nerve fibres have been proposed like high blood glucose, advanced glycation end products, sorbitol and abnormal blood fat levels and oxidative stress are considered to be important. According to unifying mechanism proposed by Brownlee – oxidative stress is the first important step leading to cellular changes discovered so far are- Increased flux through polyol pathway, production of advanced glycation end products Protein kinase activation and Increased hexosamine pathway activity which are leading to nerve damage [26].
Pharmacologic and non-pharmacologic treatments are used to reduce pain and improve physical function and improve QOL in patients with neuropathy. Many of them are associated with serious side effects.
In present study, NA appeared as a most effective drug. It has improved sensory and motor component. Glibenclamide is a commonly used sufonylurea, longer acting therefore, once daily administered for control of blood sugar level. Glibenclamide used as standard drug in the present study, which act by reducing blood sugar through increasing insulin release and also by improving peripheral utilization of glucose [27]. Pioglitazoneact by Peroxisome Proliferator-Activated Receptor Gamma (PPARγ) activation, increases glucose transport into tissue, reduces hepatic gluconeogenesis and improve utilization of glucose [28]. Epalrestat reduces aldose reductase inhibitor, helps to reduce the sorbitol levels used in diabetic neuropathy [29]. It was found to be most effective in subjects with good glycaemic control and well tolerated [30]. Hypoglycaemia with glybenclamide and weight gain, oedema, precipitation of CCF, hepatotoxicity with Pioglitazone are the common adverse effects encountered.
More effectiveness of NA indicates that it works by combined/additional mechanisms. Curcuma and Amla both individually reduce lipid perioxidation and have anti-oxidant activity [31]. NA has reduced MDA activity which has prevented lipid peroxidation and development of oxidative stress. Also, increase in the SOD and catalase levels, which have reduced superoxide radical production because of synergistic effect of combination. Therefore, reduction in oxidative stress and anti-oxidant action of NA was significantly more than any other agent. More importantly, anti-oxidant effect was seen inside the neuronal tissue indicating good penetration of NA inside the nerves. NA may have acted through multiple mechanism and showed protective effect. Additionally, it has also reduced the cholesterol and triglyceride levels. It was observed that NA in Low Dose and High Dose (LD and HD) has equivalent anti-oxidant action-indicating that increasing the dose of NA has limited advantage.
Limitation
Only anti-oxidant mechanism was studied. Further studies are required to strengthen the utlity of NA for prevention of neuropathy.
Conclusion
NA is effective in improving sensory and motor component of diabetic neuropathy. Apart from other actions, anti-oxidant action appears to be most important mechanism of action of NA. NA appears to be a potential drug formulation for preventing diabetic neuropathy.