Introduction
Heavy metals are frequently used in the preparations of traditional/folk medicines. One such preparation in Ayurveda is Nagabhasma, in which lead is the main ingredient. Lead is non-essential element to the human body and is known toxic substance to many organ systems. However, it is claimed that, the highly toxic metallic lead will be converted into health beneficial organo-metallic compound when raw lead is subjected to various traditional methods of purification during preparation as mentioned in the ancient medicinal system.
Aim
The present study is designed to evaluate the effect of such detoxification of lead in various stages of authentically prepared Nagabhasma on the learning and memory.
Materials and Methods
Using half of the human equivalent doses of traditionally prepared Nagabhasma, at intermittent stages of its preparation were fed orally to healthy Wistar rats for 30 days. After treatment, the immediate effect and residual effect after 2 months was evaluated by subjecting them to passive avoidance test. Then rats were sacrificed and hippocampus was collected for histopathological evaluation.
Results
Pure lead treated animals showed deficit in learning and memory which is indicated by spending more time in the dark compartment in passive avoidance test. However, animals treated with stage 1 to 4 Nagabhasma showed gradual increase in the memory and learning. This observation is substantiated by the findings of the histopathology of the Cornu Ammonis (CA) region of hippocampus.
Conclusion
The results of the present study indicate that, the metallic toxicity of the lead used in the preparation of bhasma was gradually decreased from stage 1 to stage 4 of preparation. Therefore, the traditional way of preparing the metallic bhasma is very critical in eliminating the possible health hazardous metallic lead toxicity.
Ayurveda, Hippocampus, Lead, Passive avoidance test
Introduction
Bhasmas are potent Ayurvedic medicaments, biologically active and powerful healing preparations. Nagabhasma is one such Ayurvedic metallic preparation in which the highly toxic lead metal undergoes a very important process called ‘bhasmikarana’ with different herbs to convert into health beneficial non-toxic form. Nagabhasma is used to treat various diseases such as diarrhoea, spleen enlargement and diabetes [1]. It has been also shown that it facilitates the formation and strengthening of the bone. It helps in faster recovery from the chorinc paralysis and also known to have beneficial role in testicular function [2].
In contrast to the health benefits of Ayurvedic Nagabhasma, it is known that lead and lead derived compounds are toxic to the normal functioning of the human body. Lead is known to alter virtually all biochemical processes and organ systems.
Symptoms may vary in adults and children; the main symptoms manifested in adults are headache, abdominal pain, memory loss, kidney failure, problems in male reproductive system and also problems in the extremities such as pain weakness and tingling sensations. In case of children, the main symptoms include loss of appetite, abdominal pain, vomiting, weight loss, constipation, anaemia, kidney failure, irritability, lethargy, learning disabilities and behaviour problems [3].
Lead is a known neurotoxicant. Thus, lead is proven to be harmful to the developing brain. There could be irreversible impairment in children’s cognitive and behavioural development as a result of early exposure. Lead exposure in young children has been linked to learning disabilities [4]. Studies have shown that children with lead poisoning have deleterious effects on the development of widespread brain areas including those implicated in cognitive, communication and social functioning [5]. Lead poisoning interferes with the normal development of a child’s brain and nervous system; therefore children are at greater risk of lead neurotoxicity than adults are [6]. Chronic adult neurotoxicity is often marked by neurocognitive and neuropsychiatric effects and impairment of short and long-term memory [7]. Over the last two decades, extensive investigation on the the neurotoxic effects of lead have been done in the animal models [8]. A study also reveals that on exposure to lead the adult rats have exhibited learning deficits [9]. Degeneration of axons and loss of myelin sheath may be observed due to lead exposure [10].
Brinck et al., observed that the pyramidal neurons of the Cornu Ammonis 1 (CA1) region and the granule cells of the fascia dentata were well preserved in the centre [11], whereas neuronal structures in outer parts were either vacuolated or hyperchromatic and shrunken. Lead treated hippocampal section showed highly vacuolated area as a layer limited to the zone of deep pyramidal layer and not the outer part of hippocampus [11].
A study on Ayurvedic herbal medicinal products in Boston, which are imported from India, revealed that 20% of them contained heavy metals [12]. In 2004 the Dutch Food and Consumer Safety Authority received reports of two cases of lead poisoning linked to the use of Ayurvedic herbal preparations from India and Nepal [13].
Many reports of the past show the toxic effect of lead in the traditional medicines such as Nagabhasma. However, we believe that, Ayurveda, an oldest and most practiced traditional medicine of Indian origin might not have overlooked at the toxicity of the lead used in this system. The stringent traditional way of preparing the Nagabhasma is the key factor which converts the toxic metal in to beneficial organo-metalic compound [14]. Therefore, the reports regarding the toxicity of lead based Ayurvedic medicines which are available in the market may be due to failure to follow the stringent traditional protocols and quality control while preparing this bhasma. Therefore, it is essential to quality control the elaborate, traditional way of purification of the lead while preparing the bhasma. Thus, this study is designed to systematically evaluate the toxic effect of lead in the Nagabhasma at various stages of its traditional purification process on learning and memory retention.
Materials and Methods
a. Nagabhasma preparation: (“Shastiputa Nagabhasma” process listed in the “grantha Ananda Kanda 2/6/25-28”.) Preparation of Nagabhasma was carried out authentically by the SDM College of Ayurveda, Udupi following the stringent steps mentioned in Rasa Ratna Samucchaya.
Preparation of Nagabhasma by following the strict and authentic traditional way includes 60 putas (steps) of purification. Out of these 60 steps, bhasma at five major steps of purification were procured. These are: a) Lead acetate (raw material used for preparation of nagabhasma); b) Stage 1 (Gomutra Shodhana); c) Stage 2 (Samanya Shodhana); d) Stage 3 (Vishesha Shodhana); and e) Stage 4 (fully processed Nagabhasma).
b. Animals: Wistar rats of both sexes weighing 100-150grams were divided into seven groups each (n=6). Following were the seven groups: a) Control; b) + Stage 1; c) + Stage 2; d) + Stage 3; e) + Stage 4 (fully prepared Nagabhasma); f) + Pure Lead used for preparation of Nagabhasma; g) + Branded – commercially available Nagabhasma.
c. Treatment plan: Two sets of experiments were carried out to check the short term effect and residual effect of the treatment of different stages of preparation of Nagabhasma.
Experiment 1: Animals underwent oral administration of half of human equivalent dose of Nagabhasma (0.15mg/100g/day)for a period of 30 days. At the end of the treatment period, the animals were subjected to behavioural test and later sacrificed to collect the brain for histopathological evaluation.
Experiment 2: Animals were orally fed with half of human equivalent dose of Nagabhasma (0.15mg/100g/day) for a period of 30 days and then left untreated for a period of two months to check the residual effect. At the end of the residual period, the animals were subjected to behavioural test and later sacrificed and brain was collected for histopathological evaluation.
d. Passive avoidance test: This behavioural test includes the learning phase and memory retention phase. On the first day the animals were familiarised with the apparatus, which consists of a large illuminated chamber and a small dark chamber. By its instinct the animals will prefer to spend more time in the dark compartment rather than the illuminated compartment. At the end of three such trials, the barrier was placed in between the chambers and a foot shock was applied to the animal in the dark compartment. On the 2nd day, the experiment was repeated without any foot shock. If the animal remembers the foot shock given in the previous day, then it will prefer to stay in the illuminated compartment rather than the dark compartment. On the other hand, if animal chooses to go to the dark compartment, then it shows that the learning and memory is hampered due to the lead present in the bhasma. In this experiment the time (in seconds) spent by the animal in the light and dark compartments were recorded.
e. Statistical analysis: The results of the passive avoidance test were analyzed using the One-way ANOVA test.
f. Histopathological analysis: After the behavioural study, animals were sacrificed and perfused with 4% formaldehyde saline and after 24hours the brain tissue was collected and kept immersed in 8% formalin for 24hour. The well-fixed brain was processed for paraffin sectioning. Briefly the brain tissues were dehydrated in ascending grades of ethyl alcohol, cleared with xylene and embedded in paraffin. Thin (5μm) coronal sections will be taken in a rotary microtome and mounted on gelatine coated glass slides. The sections were de-waxed, rehydrated and stained with cresyl violet after staining the sections were with DPX.
Results
Passive avoidance test
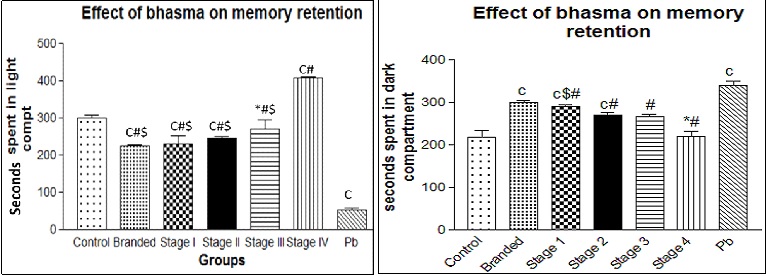
a. 30 days’ treatment: After 30 days of treatment with Nagabhasma animals were subjected to the behavioural tests. In spite of the foot shock given in the dark compartment on the previous day, the animals treated with lead acetate preferred to spend more time in the dark compartment rather than avoiding that compartment in comparison to all other test groups (p< 0.05). This shows that lead in lead acetate caused damage to hippocampus and thus impaired the learning and memory retention. On the other hand, the animals treated with fully processed Nagabhasma spent significantly less time in the dark compartment (p< 0.05) which shows that the lead in the fully processed Nagabhasma had no adverse effect on hippocampus thus memory and learning. We also observed that there was a gradual decrease in the time spent in the dark compartment by the animals treated with Stage 1 to Stage 3 of purification of bhasma. This indicates the gradual process of loss of metallic toxicity in the lead used in the preparation for bhasma from stage 1 to stage 4 [Table/Fig-1].
Graphical representation of the behavioural test after 30 days’ treatment with Nagabhasma. Statistical significance (p<0.05) is indicated in comparison with c- control, #- - Pb, * - branded bhasma, $ - stage 4 bhasma.
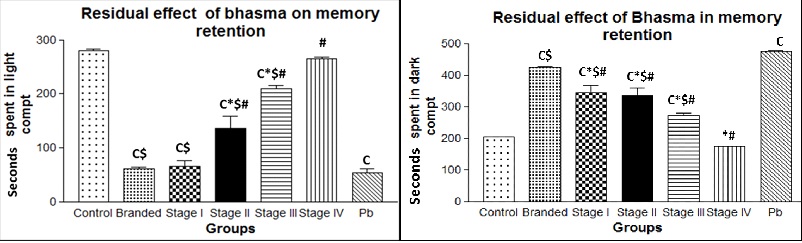
b. 30 days’ treatment and two months residual effect: After a treatment period of 30 days, the animals were left untreated for a period of two months in order to check the residual effect of lead. Even after 2 months of cessation of treatment, we observed high level of lead neurotoxicity in the pure lead and branded bhasma treated group (p<0.05) indicating the long term neurotoxic effect of metallic lead present in these test materials. However, a steady decrease in the time spent in the dark compartment by the animals treated from Stages 1 to 4 was observed which indicates the residual neurotoxic effect of lead in the early stage of preparation of bhasma. When compared to immediate effect, the residual effect seems to be intense which is indicated by fact that animals spent more time in the dark chamber in this group [Table/Fig-2].
Graphical representation of the behavioural test after 30 days’ treatment and two months residual effect. Statistical significance (p<0.05) is indicated in comparison with c - control, - Pb, * - branded bhasma, $ - stage 4 bhasma.
Histopathology of Hippocampus
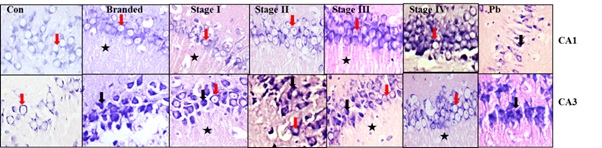
a. 30 days’ treatment: The animals treated with lead acetate shows marked reduction in the number and size of neurons. The neurons were densely stained and highly shrunken (black arrow). However, the neurons of CA1 regions of the animals treated with branded bhasma and stage 1 to 4 did not show significant changes in their structure but showed increase in the number. In comparison with the CA1 region, the neurons of CA3 region show a significant change in their structure and number. There was a marked increase in the number of neurons of the animals in all the treated groups. We also observe shrunken neurons in the animals treated with lead acetate and branded bhasma groups indicating the degenerative changes in them. On the other hand, we also observe that as the stages of purification progresses the number of healthy neurons have increased and more number of healthy neurons (red arrow) were observed in the animals treated with the fully processed (Stage 4) Nagabhasma. Thus the lead in fully processed Nagabhasma is in the non-toxic form and does not cause any alteration to the neurons of this region. Increase in the neuronal sprouts (black star) were also observed in the animals treated with branded, Stage 1, Stage 3 and Stage 4 Nagabhasma which may indicate the increased interconnections with the surrounding regions [Table/Fig-3].
Histopathology of CA1 and CA3 regions Hippocampus after 30 days’ treatment.
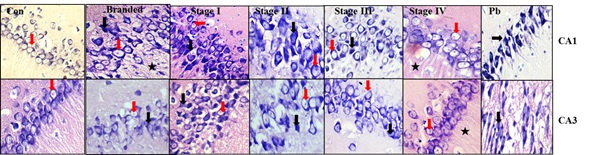
b. 30 days’ treatment and two months residual effect: After 2 months of untreated period, the neurons in the CA1 and CA3 region of the animals treated with Stages 1, 2 and pure lead showed marked degenerative changes. Most of the neurons in the CA1 and CA3 region are shrunken and darkly stained (black arrow) in animals treated with pure lead. However, stage 3 and stage 4 bhasma treated animals showed more or less healthy (red arrow) neurons which is comparable with that of control group. It also appears that, more number of neurons are seen in the CA1 region in animals treated with stage 1 to stage 4bhasma. These results indicate that, animals treated with early stages of bhasma, branded bhasma and pure lead show significant residual effect due to slow rate of efflux of the lead from the body [Table/Fig-4].
CA1 and CA3 regions of Hippocampus after 30 days’ and two months residual effect.
Discussion
Nagabhasma preparation is a very elaborate procedure. In the present study, the steps in its preparation were followed according to the “Shastiputa Nagabhasma” process listed in the “grantha Ananda Kanda 2/6/25-28”.
Even though Nagabhasma is shown to cause lead toxicity in the earlier studies, we hypothesized that, the process of making this bhasma is very crucial in removing the metallic toxicity of the lead. In addition, one study also shows that the lead in the Nagabhasma is converted into organometallic compound which may be health beneficial when processed with different herbs with stringent protocol [14]. Singh and his co-worker, earlier have shown that Nagabhasma did not alter the histology of skin, small intestine, pancreas, testis, brain, lung, kidney and liver even at the dose of 6mg/100g/day in rats [14]. However, there is no study to show the comparative study of which is available in the market and that which is prepared with all precautions to follow the stringent steps as mentioned in the ancient text. Further, as per the available literature there is not a single study to show the effect of lead in different stages of preparation of Nagabhasma on hippocampus and the memory and learning process.
The present results clearly indicate the gradual decrease in the metallic toxicity in the different stages of bhasma preparation from stage 1 to stage 4. The recommended dose of Nagabhasma for a healthy individual is 125mg/50-60kgs/day [15]. Accordingly for the rats, the dosage would be 0.25mg/100gms/day. However, in the present study a dose of 0.15mg/100g/day was given to the animals, which is much less when compared to the human equivalent dose. A previous study revealed that Nagabhasma causes cognitive dysfunctions and affects neurochemical parameters at high dosages [16].
In the present study in spite of giving half of the human equivalent dose, numerous structural changes in the neurons of the CA1 and CA3 regions of hippocampus were observed in animals fed with early stages of bhasma preparation. A dose dependent (Lead in stage 1 to stage 4) decrease in the neurotoxicity was observed in our study. This indicates the loss of metallic toxicity of lead as stage of preparation is advanced. Surprisingly, the commercially available bhasma used in this study also showed higher level of neurotoxicity indicating the improper processing of lead during its preparation. Our study also reveals that, residual effect (long term effect) of lead is more intense than immediate effect which is indicated by the time spent in the dark compartment. Animals in the residual group spent more time in the dark compartment than the animals tested immediately after one month of treatment. However, histological analysis does not show same effect. The hippocampal region of animals in the residual group shown healthier neurons than the animals tested for immediate effect. To explain this phenomenon further analysis of complicated internal circuit, synapsis and neurotransmitter may be warranted.
Therefore, the non-toxic form of lead in the fully processed Nagabhasma may be attributed to the organic molecules used in the process of making the bhasma such as turmeric powder, vaasa, neem leaves and nirgundi which play an important role to increase the efficacy of bhasma [14]. A process called ‘bhasmikarana’ converts the toxic metal in to a highly oxidised form to render a high medicinal value [17]. Lead in Nagabhasma is found to be in the nano-crystalline lead sulphide form [14]. These nanoscale materials are small enough to enter cells and organelles [18].
Thus, the toxic metallic form of lead used to prepare the Nagabhasma gets converted into a non-toxic organo-metallic compound easily acceptable by the body.
Limitation
Further research is required to analyse the exact changes in the physical and chemical structure of the metallic lead into non-toxic form. Additionally, understanding the process of modification of lead into substance like nano-particle would be of great help in designing new drug delivery system also.
Conclusion
In our study, we conclude that lead in the Nagabhasma when prepared by following the stringent methods of preparation is highly acceptable to the body. The lead metal loses its toxicity gradually as the steps of purification proceeds from the crude form of lead to the fully processed Nagabhasma. Further, cellular and molecular analysis may help us to understand the exact transformation of highly toxic metallic lead into non-neurotoxic form of lead and its actual role in neurocognition. Understanding the alterations in the metallic composition in the led may further help us to clearly understand the non-toxic nature of the nagabhasma.
[1]. Kulkarni-Dudhgaonkar SB, RasaratnaSamuchyaya 1970 KolhapurShivaji University Publication:158 [Google Scholar]
[2]. Wadekar M, Gogte V, Khandagale P, Prabhune A, Comparative study of some commercial samples of NagabhasmaAncient Science of Life 2004 23(4):48-58. [Google Scholar]
[3]. Pearce JMS, Burton’s line in lead poisoningEur Neurol 2007 57(2):118-19. [Google Scholar]
[4]. Barrett JR, Sex-specific cognitive effects of leadEnviron Health Perspect 2009 117(9):390-93. [Google Scholar]
[5]. Lidsky TI, Schneider JS, Lead neurotpxicity in children: basic mechanisms and clinical correlatesBrain 2003 126:5-19. [Google Scholar]
[6]. Sanders T, Liu Y, Buchner V, Tchounwou PB, Neurotoxic effects and biomarkers of lead exposure: A reviewRev Environ Health 2009 24(1):15-45. [Google Scholar]
[7]. Schwartz J, Low-level lead exposure and children’s IQ: a meta-analysis and search for a thresholdEnviron Res 1994 65(1):42-55. [Google Scholar]
[8]. Bais SK, Chandewar AV, Significance of some to toxicological parameters standardization of herbal medicine marketed in India: A reviewJ Pharm Biomed Sci 2010 7(5):1-4. [Google Scholar]
[9]. Kuhlmann AC, McGlothan JL, Guilarte TR, Developmental lead exposure causes spatial learning deficits in adult ratsNeurosci Lett 1997 233(2-3):101-04. [Google Scholar]
[10]. Karri SK, Saper RB, Kales SN, Lead encephalopathy due to traditional medicinesCurr Drug Saf 2008 3(1):54-59. [Google Scholar]
[11]. Brinck U, Wechsler W, Microscopic examination of hippocampal slices after shortterm lead exposure invitroNeurotoxicolTeratol 1985 11(6):539-43. [Google Scholar]
[12]. Saper RB, Kales SN, Paquin J, Burns MJ, Eisenberg DM, Davis RB, Phillips RS, Heavy metal content of ayurvedic herbal medicine productsJAMA 2004 292(23):2868-73. [Google Scholar]
[13]. Kanen BL, Perenboom RM, Chronic lead intoxication associated with Ayurvedic medicationNed TijdschrGeneeskd 2005 149(52):2893-96. [Google Scholar]
[14]. Singh SK, Gautam DN, Kumar M, Rai SB, Synthesis, characterization and histopathological study of a lead-based Indian traditional drug: bhasmanagabhasmaIndian J Pharm Sci 2010 72(1):24-30. [Google Scholar]
[15]. Sarkar PK, Das S, Prajapati PK, Ancient concept of metal pharmacology based on Ayurvedic literatureAncient Sci Life 2010 29(4):1-6. [Google Scholar]
[16]. Gyan VM, Vohora SB, Effects of some indigenous Indian medicinal preparations on cognitive functionsAsian J Exp Sci 2001 15(1&2):27-37. [Google Scholar]
[17]. Wadekar MP, Rode CV, Bendale YN, Patil KR, Prabhune AA, Preparation and characterization of a copper based Indian traditional drug: TambrabhasmaJ Pharm Biomed Anal 2005 39(5):951-55. [Google Scholar]
[18]. Klein J, Probing the interactions of proteins and nanoparticlesProc Natl Acad Sci U S A 2007 104(7):2029-30. [Google Scholar]