Decidual CD56+ Natural Killer Cells in Spontaneous Early Pregnancy Loss- An Immunohistochemical Study
Balamurugan Senthilnayagam1, Sridhar Karthikeyan2, Jayapriya Sukumaran3, Anoop Srivalsan4, Ramesh Rao5, Vasantha Subbiah6
1 Professor, Department of Pathology, Chettinad Hospital and Research Institute, Kancheepuram, Tamil Nadu, India.
2 Intern, Department of Pathology, Chettinad Hospital and Research Institute, Kancheepuram, Tamil Nadu, India.
3 Assistant Professor (Formerly), Department of Microbiology, Chettinad Hospital and Research Institute, Kancheepuram, Tami Nadu, India.
4 Professor, Department of Obstetrics and Gynaecology, Chettinad Hospital and Research Institute, Kancheepuram, Tamil Nadu, India.
5 Professor, Department of Pathology, Chettinad Hospital and Research Institute, Kancheepuram, Tamil Nadu, India.
6 Professor, Department of Obstetrics and Gynaecology, Chettinad Hospital and Research Institute, Kancheepuram, Tamil Nadu, India.
NAME, ADDRESS, E-MAIL ID OF THE CORRESPONDING AUTHOR: Dr. Balamurugan Senthilnayagam, 3D KG Traditions 1, North Gopalapuram First Street, Chennai-600086, Tamil Nadu, India.
E-mail: ambikayal@yahoo.co.in
Introduction
Natural killer cells are believed to promote placental and trophoblastic growth and provide immune- modulation at maternal-fetal interface in pregnancy and their role in reproductive failure has been a matter of discussion.
Aim
To study CD56+ Natural killer cells in spontaneous pregnancy loss.
Materials and Methods
In this prospective observational study, formalin-fixed paraffin embedded sections from products of conception from twenty women each with spontaneous early pregnancy loss (test group) and elective pregnancy termination (control group). Immunohistochemical staining with CD 56 monoclonal antibody was done by avidin-biotin peroxidase technique. CD56+ cells in decidua were counted under light microscopy by two independent observers in ten high power fields (40X) and mean cell count taken. Student’s paired ‘t’-test was used to statistically compare CD56+ NK cell population between the test and control groups.
Results
The mean number of CD56+ NK cells was higher in the decidual tissue of women who had spontaneous early pregnancy loss (mean±SD, 57.55±1.79) as compared to the mean number of CD56+ NK cells in the decidual tissue from women who underwent elective termination (mean±SD, 50.9±3.46). The difference was statistically significant (difference of 6.65 with 95% confidence interval of 4.76 to 8.54, p-value <0.0001).
Conclusion
This could imply that CD56+ NK cells have a role in the pathogenesis of spontaneous early pregnancy loss and further large scale studies can throw more light on the mechanism and designing of appropriate therapy.
Immunity, Reproductive failure, Trophoblastic growth
Introduction
Immunological factors are implicated in the aetiopathogenesis of reproductive failure. Recent interest is on the role of Natural Killer (NK) cells in pregnancy failure. In normal pregnancy, NK cells are thought to promote placental and trophoblastic growth and provide immune-modulation at the maternal-fetal interface and these NK cells are believed to be dysregulated in unexplained recurrent pregnancy loss. In recent years, the role of NK cells as a significant factor of reproductive failure has been a subject of discussion [1–3]. The aim was to demonstrate and quantitate the CD56 positive Natural killer (NK) cells in the decidual tissue from women who had spontaneous early pregnancy loss and compare the same with that from the gestational age-matched control decidua from women who had elective pregnancy termination.
Materials and Methods
Twenty women presenting with spontaneous early pregnancy loss were recruited for this prospective observational study during six month period (January–June 2013) at Chettinad Hospital & Research Institute, Tamil Nadu, India. Women with known uterine abnormalities and infections were excluded. Gestational age-matched (9-13 weeks) twenty women without any systemic illness attending the hospital for elective pregnancy termination formed the control group. Institutional ethics committee approval and informed consent from study subjects were obtained prior to start of the study.
Products of conception sent for histopathology examination were formalin-fixed, processed, paraffin embedded and 3-4μ thick sections. In addition to routine haematoxylin & eosin staining, immuno-staining for CD56 marker was performed using Novacastra NCL-L CD56-1B6 in the dilution of 1:50 for 60 minutes followed by secondary antibody and enzyme-chromogen complex. Negative reagent control (by omitting primary antibody) and positive tissue control (cerebellum) were run for quality assurance. Positive cells [Table/Fig-1,2 and 3] were counted as described by Tuckerman et al., in 10 non- overlapping high power fields (40X) under light microscope by two independent observers and mean count was taken as the final result [4]. Student paired ‘t’-test was used to statistically compare the mean CD56+ cell counts of test and control groups. The GraphPad online statistical calculator was used. The p-value ≤ 0.05 was considered statistically significant.
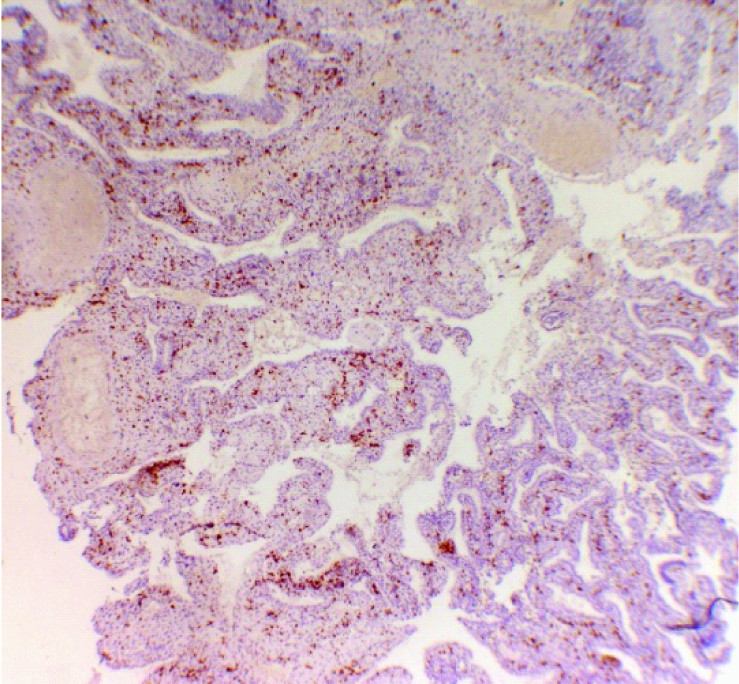
Low power view- CD56 positivity in decidua from women with spontaneous early pregnancy loss. (CD56 immunostain, 10X).
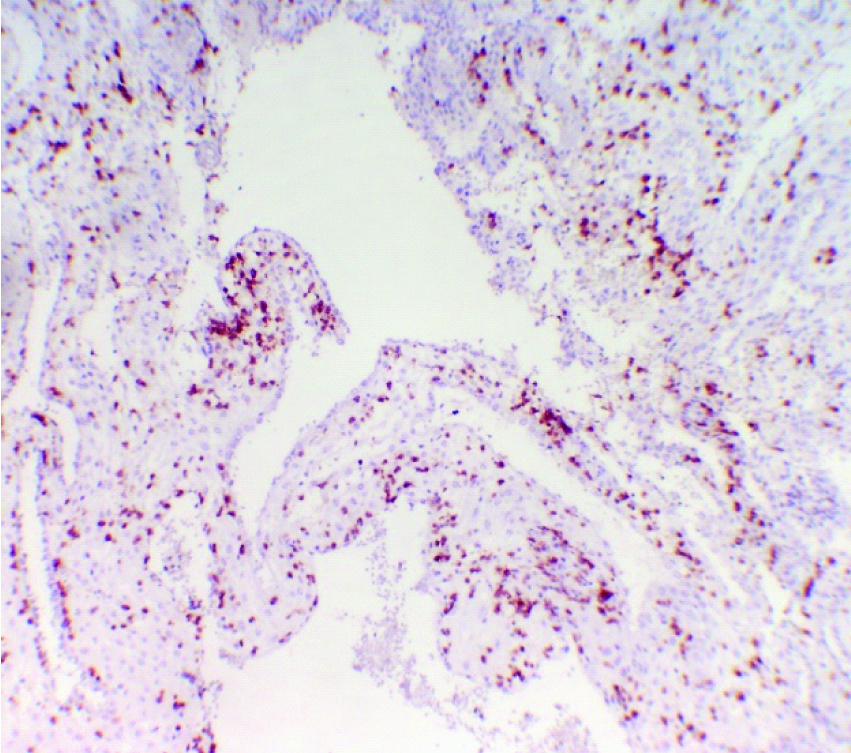
High power view – CD56 positivity in deciduas from women with spontaneous early pregnancy loss. (CD56 immunostain, 40X).
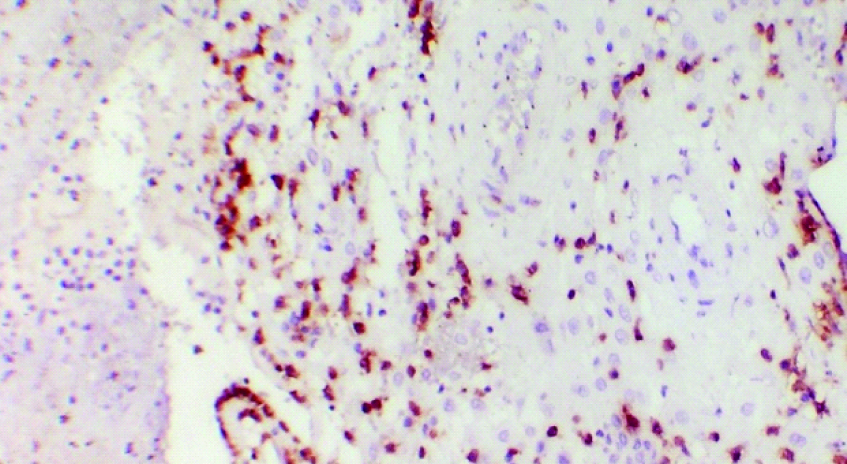
CD56 positivity in decidua from women with elective pregnancy termination. (CD56 immunostain,40X).
Results
The age of the study group ranged from 20 to 32 years (mean±SD, 25±3.32) and gestational age ranged from 9 to 13 weeks (mean±SD, 10.85±1.23) of pregnancy. CD56+ cells were membrane stained and found distributed in the decidua of both spontaneous pregnancy loss (test) group and medical termination of pregnancy (control) group [Table/Fig-1,2 and 3]. The number of CD56+ cells per high power field (40X) ranged from 54 to 62 (mean±SD, 57.55±1.79) in the test group and 45 to 58 (mean±SD 50.9±3.46) in the control group. The mean number of CD56+ NK cells was higher (difference of 6.65 with 95% confidence interval of 4.76 to 8.54) in the decidual tissue of women who had spontaneous early pregnancy loss (test group) as compared to the mean number of CD56+ NK cells in the decidual tissue from women who underwent elective termination (control group). The difference was statistically significant with p-value <0.0001 [Table/Fig-4].
Results of student’s paired ‘t’-test comparing CD56+ cell counts of test group with control group.
| Study Group-Spontaneous Early Pregnancy Loss | Control Group-Elective Termination Pregnancy | Difference Between Groups (with 95% Confidence Intervals) | p-value |
|---|
| Mean | 57.55 | 50.90 | 6.65 (4.76- 8.54) | p< 0.0001 |
| Standard Deviation | 1.79 | 3.46 |
| Standard Error of Means | 0.40 | 0.77 |
| Number of Subjects | 20 | 20 |
Discussion
The Uterine NK (uNK) cells play a pivotal role in the normal decidual angiogenesis and spiral arteriole remodelling and these cells secrete cytokines that may influence the decidual and trophoblast microenvironment. The mRNAs for granulocyte CSF (Colony Stimulating Factor), M-CSF (Macrophage-Colony Stimulating Factor), GM-CSF (Granulocyte Macrophage-Colony Stimulating Factor), TNF-α (Tumor Necrosis Factor - α), IFN-γ (Interferron- γ), TGF-β (Tumor Growth Factor- β) and LIF (Leukaemia Inhibitory Factor) have been found in human decidual CD56+ cells and receptors for GM-CSF, CSF-1, IFN-γ, and TNF-α have been demonstrated on human trophoblast cells, arguing for a role of uNK cell-derived cytokines on trophoblast growth and differentiation [5,6]. Laird SM et al., in a review of immune cells reported differences in the CD56+ cells and cytokine profile in spontaneous pregnancy loss compared to healthy pregnancy [7]. This probably suggests that NK cells may have a role in spontaneous pregnancy loss and further studies are needed to unravel the under lying mechanisms.
Our study found an increase in the mean number of CD56+ cells in spontaneous pregnancy loss. This finding is consistent with other authors. Clifford K et al., reported increased mean numbers of CD56+ cells in the endometrium of women with recurrent early miscarriage [8,9]. Coulam et al., and Yamada et al., reported high CD56+ NK cell number and activity in Recurrent miscarriage women with chromosomally normal fetuses, thus suggesting that the presence of high CD56+ levels are a cause rather than an effect of recurrent miscarriage [10]. Similar observations were made by other authors like Emmer PM et al., and Papamitsou et al., in tissues from spontaneous pregnancy loss and curettage of non-vital pregnancy and Quenby et al., in luteal endometrial biopsies from women with unexplained recurrent pregnancy loss [11–13].
In flow cytometric analyses, two CD56+ cell subsets (CD56 bright + & CD56dim +) have been described and Lachapelle et al., observed a greater percentage of CD16+ CD56dim +cells and smaller percentage of CD16+ CD56bright + cells in luteal phase endometrial biopsies of recurrent pregnancy loss women [14,15]. Vassiliadou and Bulmer reported a decreased cytotoxic capability of decidual CD56+ NK cells in placental tissue from spontaneous aborters [16]. However, the difference in immunophenotypic profile of NK cells or functional dysregulation of NK cells were beyond our scope of the study.
Certain other authors observed no difference in the NK cell number or percentage in endometrial biopsies (Shimada et al., and Michimata et al.,) or in placental tissue from spontaneous miscarriages (Yamamoto et al., and Quack et al.,) [17–20]. This may be related to the differences in the tissues studied (frozen or paraffin) and the technique used (flow cytometry or immunohistochemistry).
Tang et al., reported that prednisolone decreased uNK cell number and the treated women subsequently had a successful pregnancy in clinical trials initiated to examine prednisolone as an intervention to decrease uNK cell number and consequently recurrent spontaneous abortion [21].
Limitation
Our sample size was limited as this was a short term study and the functional activity of CD56+ NK cells could not be studied which is a scope for future research.
Conclusion
It is likely that NK cells have a role in spontaneous pregnancy loss although the mechanisms are not yet clear. Future large scale studies may throw more light on this. A comprehensive understanding of the immune mechanism underlying early pregnancy loss shall help us to design specific and effective therapy for such patients.
[1]. Tang AW, Alfirevic Z, Quenby S, Natural killer cells and pregnancy outcomes in women with recurrent miscarriage and infertility: a systematic reviewHum Reprod 2011 26(8):1971-80. [Google Scholar]
[2]. Seshadri S, Sunkara SK, Natural killer cells in female infertility and recurrent miscarriage: a systematic review and meta-analysisHum Reprod Update 2014 20(3):429-38. [Google Scholar]
[3]. Jabrane-Ferrat N, Siewiera J, The up side of decidual natural killer cells: new developments in immunology of pregnancyImmunology 2014 141(4):490-97. [Google Scholar]
[4]. Tuckerman E, Laird SM, Prakash A, Li TC, Prognostic value of the measurement of uterine natural killer cells in the endometrium of women with recurrent miscarriageHum Reprod 2007 22:2208-13. [Google Scholar]
[5]. Vinketova K, Mourdjeva M, Oreshkova T, Human decidual stromal cells as a component of the implantation niche and a modulator of maternal immunityJournal of Pregnancy 2016 2016:8689436 [Google Scholar]
[6]. Rätsep MT, Felker AM, Kay VR, Tolusso L, Hofmann AP, Croy BA, Uterine natural killer cells: supervisors of vasculature construction in early decidua basalisReproduction 2015 149(2):91-102. [Google Scholar]
[7]. Laird SM, Tuckerman EM, Cork BA, Linjawi S, Blakemore AIF, Li TC, A review of immune cells and molecules in women with recurrent miscarriageHuman Reproduction Update 2003 9(2):163-74. [Google Scholar]
[8]. Clifford K, Flanagan AM, Regan L, Endometrial CD56+ natural killer cells in women with recurrent miscarriage: a histomorphometric studyHum Reprod 1999 14(11):2727-30. [Google Scholar]
[9]. Yamada H, Kato EH, Kobashi G, Ebina Y, Shimada S, Morikawa M, High NK cell activity in early pregnancy correlates with subsequent abortion with normal chromosomes in women with recurrent abortionAm J Reprod Immunol 2001 46:132-36. [Google Scholar]
[10]. Coulam CB, Goodman C, Roussev RG, Thomason EJ, Beaman KD, Systemic CD56+ cells can predict pregnancy outcomeAm J Reprod Immunol 1995 33:40-46. [Google Scholar]
[11]. Emmer PM, Steegers EA, Kerstens HM, Bulten J, Nelen WL, Boer K, Altered phenotype of HLA-G expressing trophoblast and decidual natural killer cells in pathological pregnanciesHum Reprod 2002 17:1072-80. [Google Scholar]
[12]. Papamitsou T, Toskas A, Papadopoulou K, Sioga A, Lakis S, Chatzistamatiou M, Immunohistochemical study of immunological markers: HLAG, CD16, CD25, CD56 and CD68 in placenta tissues in recurrent pregnancy lossHistol Histopathol 2014 29(8):1047-55. [Google Scholar]
[13]. Quenby S, Farquharson R, Uterine natural killer cells, implantation failure and recurrent miscarriageReproductive Bio Medicine Online 2006 13(1):24-28. [Google Scholar]
[14]. Giuliani E, Parkin KL, Lessey BA, Young SL, Fazleabas AT, Characterization of uterine NK cells in women with infertility or recurrent pregnancy loss and associated endometriosisAmerican journal of reproductive immunology 2014 72(3):262-69. [Google Scholar]
[15]. Lachapelle MH, Miron P, Hemmings R, Roy DC, Endometrial T, B, and NK cells in patients with recurrent spontaneous abortion. Altered profile and pregnancy outcomeJ Immunol 1996 156(10):4027-34. [Google Scholar]
[16]. Vassiliadou N, Bulmer JN, Expression of CD69 activation marker by endometrial granulated lymphocytes throughout the menstrual cycle and in early pregnancyImmunology 1998 94:368-75. [Google Scholar]
[17]. Shimada S, Kato EH, Morikawa M, Iwabuchi K, Nishida R, Kishi R, No difference in natural killer or natural killer T-cell population, but aberrant T-helper cell population in the endometrium of women with repeated miscarriageHum Reprod 2004 19:1018-24. [Google Scholar]
[18]. Michimata T, Ogasawara MS, Tsuda H, Suzumori K, Aoki K, Sakai M, Distributions of endometrial NK cells, B cells, T cells, and Th2/Tc2 cells fail to predict pregnancy outcome following recurrent abortionAm J Reprod Immunol 2002 47:196-202. [Google Scholar]
[19]. Yamamoto T, Takahashi Y, Kase N, Mori H, Role of decidual natural killer cells in patients with missed abortion: differences between cases with normal and abnormal chromosomeClin Exp Immunol 1999 116:449-52. [Google Scholar]
[20]. Quack KC, Vassiliadou N, Pudney J, Anderson DJ, Hill JA, Leukocyte activation in the decidua of chromosomally normal and abnormal fetuses from women with recurrent abortionHum Reprod 2001 16:949-55. [Google Scholar]
[21]. Tang A-W, Alfirevic Z, Turner MA, Drury J, Quenby S, Prednisolone Trial: Study protocol for a randomised controlled trial of prednisolone for women with idiopathic recurrent miscarriage and raised levels of uterine natural killer (uNK) cells in the endometriumTrials 2009 10:102 [Google Scholar]