Hypersensitivity with Inhalational Budesonide: An Under Recognised Entity
Pramod Kumar Sharma1, Neeraj Gupta2, Najmul Hasan3, Bhaskar Krishnamurthy4, Surjit Singh5
1 Associate Professor, Department of Pharmacology, AIIMS, Jodhpur, Rajasthan, India.
2 Associate Professor, Department of Paediatrics, AIIMS, Jodhpur, Rajasthan, India.
3 Tutor, Department of Pharmacology, AIIMS, Jodhpur, Rajasthan, India.
4 Senior Resident, Department of Pharmacology, AIIMS, Jodhpur, Rajasthan, India.
5 Assistant Professor, Department of Pharmacology, AIIMS, Jodhpur, Rajasthan, India.
NAME, ADDRESS, E-MAIL ID OF THE CORRESPONDING AUTHOR: Dr. Neeraj Gupta, Associate Professor, Department of Paediatrics, AIIMS, Jodhpur-342005, Rajasthan, India.
E-mail: neerajpgi@yahoo.co.in
Hypersensitivity reactions are commonly encountered with drugs such as beta lactams, sulphonamides, allopurinol etc., Corticosteroids are frequently employed in the treatment of drug induced allergic reactions. Therefore, it is highly unlikely that a corticosteroid itself may cause such a reaction as an adverse effect. We had encountered a rare case of hypersensitivity reaction with inhalational budesonide in an eight-year-old boy. The patient developed maculopapular rashes over the back, buttocks and legs accompanied with pruritus within four hours of administration of the first dose. The reaction subsided within two days on withdrawal of the drug and treatment with oral fexofenadine. Re-introduction of budesonide by the same route after a month resulted in appearance of similar reaction. Both the parents of the patient were known cases of allergic rhinitis suggesting allergic pre-disposition in the family. Causality analysis using WHO-UMC scale suggested certain association of this allergic reaction with inhaled budesonide.
Corticosteroids, Maculopapular rash, Pruritus
Case Report
An eight-year old boy weighing 42 kg, presented with history of recurrent episodes of cough associated with difficulty in breathing and whistling sounds since last two years. These episodes were more during winter season. Patient had visited multiple physicians and medical records revealed treatment in form of nebulization (salbutamol and budesonide) and oral drugs including prednisolone, antihistaminics and montelukast. There was marked improvement in symptoms following nebulizations. The exact frequency of budesonide nebulization could not be elicited from history or from medical records. Moreover, parents also ruled out occurrence of any drug reaction in general and subsequent to nebulization in particular. The parents of the patient were suffering from seasonal allergic rhinitis.
The patient had presented with similar symptoms associated with mild tachypnea and bilateral expiratory wheeze. The chest radiograph revealed bilateral hyperinflation and thus, diagnosis of bronchial asthma was made. The patient was advised to start inhalational salbutamol and budesonide {2 puffs of 100μg each through Metered Dose Inhaler (MDI) and spacer}.
A month later, the same child presented with enteric fever like symptoms for which he was hospitalized. General and physical examination at the time of hospital admission were non-significant except for pyrexia. The respiratory system examination revealed bilateral crepitations and occasional wheezing. With the exception of an elevated lymphocyte count (59%), investigations were unremarkable. The parents informed that within 4 hours of first inhalation of budesonide at home, the child developed pruritic maculopapular rashes that evolved extensively over next 12 hours to involve entire back, buttocks and legs [Table/Fig-1,2]. The rash was of mild to moderate severity and apparently did not involve mucous membrane. The reaction subsided within two days upon withdrawal of the drug and treatment with oral fexofenadine. The child was not on regular medication for his underlying problem of bronchial asthma since then.
Lesion involving back and buttocks.
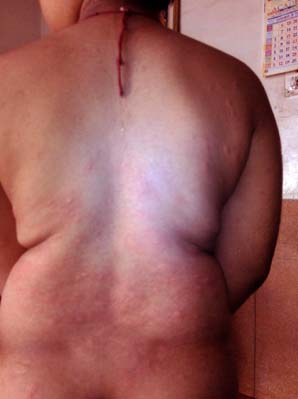
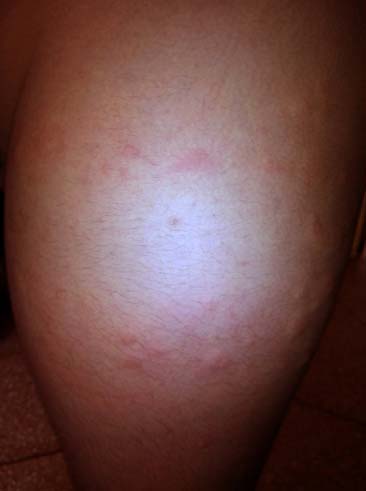
The nebulization with salbutamol (2.5mg) was initiated along with specific treatment for enteric fever. The administration of budesonide was reconsidered in view of occurrence of rash at home without any physical verification, rarity of such event, being under medical supervision and history of budesonide administration in past as per medical records. The child was administered budesonide (2 puffs of 100μg each through MDI) and he developed maculopapular rash, urticaria and pruritus within 4-6 hours similar to previous episode. The rash disappeared within 24 hours of onset. Budesonide was stopped and patient continued on salbutamol. The patient also received fexofenadine for symptomatic relief. Patient was started on MDI fluticasone (2 puff of 125μg twice daily). There was no recurrence of rash.
Analysis by WHO-UMC causality scale indicated that the reaction is certainly associated with budesonide as the reaction occurred with reasonable time relationship to the administration [1]. Also, withdrawal of the drug led to disappearance of symptoms and reintroduction resulted in reappearance of the symptoms (positive de-challenge and re-challenge).
The written informed consent was obtained from the parents for publishing medical details and photographs of the patient. Parents were assured that publication is only for research and scientific purpose and will not affect privacy and confidentiality of the patient.
Discussion
Prevalence of allergic reactions to corticosteroids is quite variable. Differences in prescribing habits, awareness of corticosteroid allergy among medical professionals and diagnostic procedures, may have variable influence. Because of resemblance to the symptomatology of the underlying disease, corticosteroids induced hypersensitivity reactions remain largely undiagnosed. Immediate type hypersensitivity reactions are characterized by urticaria, angioedema and anaphylaxis appearing within 1 hour of drug intake and are most likely to be mediated by specific IgE antibodies. Among corticosteroids, methylprednisolone and hydrocortisone are frequent causes of such reactions. Non-immediate reactions are T-cell mediated and symptoms are usually delayed up to 48 hours [2]. Hypersensitivity reactions are more frequent with local application but usually are of lesser severity than those caused by systemic administration [2]. The reactions have been more commonly reported with the non-fluorinated steroids than the fluorinated ones (e.g., dexamethasone or betamethasone) [3].
Bundgaard, has suggested that corticosteroids are degraded to a corticosteroid glyoxol that then reacts with arginine molecules of proteins to form the complete antigen [4]. The non-fluorinated compounds, which degrade and react with arginine more rapidly, are more prone to cause such reactions. Hypersensitivity reactions produced by corticosteroid are mainly type I and type IV immunological mechanisms as described by Gell and Coombs [5,6]. Urticaria and maculopapular exanthema are common manifestations of type IV reaction. Symptoms observed in this case were different from symptoms reported with inhalational budesonide in a previous study i.e., dry cough, dyspnoea and glossopharyngeal oedema [7]. The patient presented predominantly with generalized skin manifestations a rare occurrence immediately after first budesonide inhalation. In a study of 51 asthmatic patients those who were on chronic inhaled corticosteroids therapy, only one patient got allergic skin symptoms within 2 days after starting the therapy [8]. Moreover, re-appearance of similar episode on re-administration prompted us to report this case. Such reactions may be classic allergic or pseudoallergic in nature and may require objective tests such as patch testing, provocative challenges for diagnosis. However, skin testing is more useful in diagnosing allergy in those with a clear history of immediate hypersensitivity to corticosteroids [9].
We presume that, predisposition of the patient to the allergy as suggested by family history might have made him vulnerable to such a reaction. Such reactions usually occur more frequently in asthmatic subjects [10,11] or in those being regularly treated with systemic Corticosteriods [12]. However, the role of genetic pre-disposition in the development of allergy to budesonide cannot be ruled out.
Conclusion
Diagnosing hypersensitivity reactions with corticosteroids remains a challenge. Failure to detect such events has undoubtedly resulted in under-reporting. Hypersensitivity reactions can be induced by all types of corticosteroid formulations i.e., oral, parenteral, nasal and inhaled. Clinician should be able to recognize and be aware of the potential for hypersensitivity reaction with inhalational budesonide. A thorough and close evaluation of the case is essentially required to label an adverse drug effect as a true hypersensitivity reaction. This may help them substitute causative steroid with an alternative preparation which can be tolerated by the patient. Suspicion and due consideration to the possibility of hypersensitivity reactions with steroids is needed in routine clinical practice.
[1]. The use of the WHO-UMC system for standardised case causality assessment. http://who-umc.org/Graphics/24734.pdf [Google Scholar]
[2]. Torres MJ, Canto G, Hypersensitivity reactions to corticosteroidsCurr Opin Allergy Clin Immunol 2010 10(4):273-79. [Google Scholar]
[3]. Venturini M, Lobera T, del Pozo MD, Gonzalez I, Blasco A, Immediate hypersensitivity to corticosteroidsJ Investig Allergol Clin Immunol 2006 16:51-56. [Google Scholar]
[4]. Bundgaard H, The possible implication of steroid-glyoxal degradation products in allergic reactions to corticosteroidsArch Pharm Chem Sci Ed 1980 8:83-90. [Google Scholar]
[5]. Mansfield LE, Ting S, Haverly RW, Anaphylaxis caused by the sodium succinate ester of hydrocortisone and methylprednisoloneJ Asthma 1986 23:81-83. [Google Scholar]
[6]. Nucera E, Buonomo A, Pollastrini E, A case of cutaneous delayed-type allergy to oral dexamethasone and to betamethasoneDermatology 2002 2049(3):248-50. [Google Scholar]
[7]. Garcia-Abujeta JL, Fernandez L, Maquiera E, Picans I, Rodriguez F, Jerez J, Contact allergy to budesonide in an oral sprayContact Dermatitis 1995 32:253 [Google Scholar]
[8]. Kilpio K, Hannuksela M, Corticosteroid allergy in asthmaAllergy 2003 58:1131-35. [Google Scholar]
[9]. Baker A, Empson M, The R, Fitzharris P, Skin testing for immediate hypersensitivity to corticosteroids: a case series and literature reviewClin Exp Allergy 2015 45:669-76. [Google Scholar]
[10]. Karsh J, Yang WH, An anaphylactic reaction to intra-articular triamcinolone: a case report and review of the literatureAnn Allergy Asthma Immunol 2003 90:254-58. [Google Scholar]
[11]. Nakamura H, Matsuse H, Obase Y, Mitsuta K, Tomari S, Saeki S, Clinical evaluation of anaphylactic reactions to intravenous corticosteroids in adult asthmaticsRespiration 2002 69:309-13. [Google Scholar]
[12]. Kamm GL, Hagmeyer KO, Allergic type reactions to corticosteroidsAnn Pharmacother 1999 33:451-60. [Google Scholar]