Intravenous Regional Anaesthesia (IVRA) is known to be a reliable, simple and a cost-effective method of providing anaesthesia for minor surgical procedures to the extremities [1]. Limitation of this block include tourniquet discomfort, short duration of block and absence of post-operative analgesia. To mitigate these effects and improve the quality of the block various drugs such as NSAID’s like lornoxicam [2], opioids [3], ketorolac [4], neostigmine [5] and muscle relaxants [6] have been added. But various side effects such as delayed respiratory depression, pruritis, nausea and very short duration of analgesia after release of tourniquet have necessitated continued study of newer additives to the block.
Materials and Methods
This study was a randomized, prospective controlled double blinded study. It was done at a tertiary hospital from January 2014 to January 2015 after approval from the Medical Ethics Committee. The study population consisted of 60 indoor patients of ASA physical status I and II of either sex, between the ages of 18 to 60 years, scheduled for orthopaedic surgery of the upper extremity. Pre-operative evaluation included history, general physical examination and routine investigations. Patients were explained the procedure and the Visual Analogue Scale Scoring system (VAS) wherein the patient was asked to grade his/her pain on a numeric scale of 0 to 10 (where 0 = no pain present and a score of 10=the worst pain) pre-operatively and a written informed consent was obtained. Those with Raynaud’s disease, sickle cell anaemia, and peripheral vascular disease or known allergy to either drug were excluded from the study.
The study population was randomized into two groups of 30 each by closed envelope technique; Group C (Control Group) received 40ml 0.5% lignocaine solution and Group D (Dexmedetomidine group) received 0.5μg/kg of dexmedetomidine added to 0.5% lignocaine to a total volume of 40ml. The desired 0.5% concentration was prepared by adding 30ml of 0.9% normal saline to 10ml of 2% preservative free lignocaine. No pre-medication was administered. After measuring the patient’s weight, electrocardiogram, pulse oximetry and non-invasive blood pressure was monitored in all patients.
Before establishing the anaesthetic block, two intravenous can-nulae were secured. One 22G cannula was secured in a vein on the dorsum of the operative hand distal to the operative site and the other 20G cannula in the opposite hand for fluid infusion. Ringer lactate was infused at the rate of 2ml/kg/hour. The operative arm was elevated for 3 minutes above the level of the heart to facilitate venous drainage by gravity and then exsanguinated with an Esmarch bandage.
A double cuffed pneumatic tourniquet was placed around the upper arm. The proximal cuff was inflated to 100mmHg above the systolic BP of the patient. The Esmarch bandage was removed after tourniquet application. The tourniquet inflation time was noted. Loss of waveform of the pulse oximeter, with loss of radial pulse demonstrated that the arm was satisfactorily isolated from the circulation.
The solution was injected over 90 seconds by an anaesthesiologist blinded to the exact composition of the injected drugs. After the injection the cannula was removed with strict asepsis and pressure applied over site until bleeding ceased. The various parameters that were studied were assessed by a different anaesthesiologist.
Sensory block was assessed by a pin prick performed with a 25 Gauge short bevelled hypodermic needle once every 30 seconds. Subject response was evaluated in the dermatomes with the sensory distribution of medial and lateral ante-brachial cutaneous, ulnar, median and radial nerve and the time for onset of analgesia noted. Motor block was assessed by asking the subject to flex and extend his/her wrist and fingers every 30 seconds until weakness of movement were confirmed. After confirming that both sensory and motor anaesthesia had been achieved, the distal tourniquet cuff was inflated to 250mmHg, and the proximal tourniquet cuff was released, then the surgery was commenced. The surgery was started only after sensory block was achieved. If a patient had no sensory or motor block it was considered a failure of block and the patient was administered general anaesthesia.
Intra-operative boluses of IV fentanyl 1μg/kg were given for treatment of tourniquet pain (VAS>3), and the total intraoperative fentanyl consumption was noted. Tourniquet was deflated following a minimum of 30 minutes after inflation (it was not kept inflated for >90 min). At the end of surgery the tourniquet was deflated by cyclic deflation technique, sensory and motor recovery times were also noted after tourniquet deflation. Onset of sensory blockade was defined as the time taken from the completion of the injection of the study drug until the subject did not feel the pin prick in any of the dermatomes. Recovery of sensory block defined as time from release of tourniquet to the recovery of sensation. Onset of motor block was from the time of injection of study drug till the subject had no voluntary movement of fingers and wrist. Recovery of motor block was noted as the time from deflation of tourniquet to movement of wrist and fingers. VAS and Sedation Scores were noted at 0, 5, 10, 15, 20, 25, 40, and 60 min following tourniquet inflation and at 30mins, 2, 4, 6, 12 and 24 hours after the tourniquet deflation. Sedation score was based on a numerical rating scale of 1 to 5(1-Completely awake, 2-Awake but drowsy, 3-Asleep but responsive to verbal commands, 4-Asleep but responsive to tactile stimulus and 5-Asleep but not responsive to any stimulus) [10].
Postoperatively patients were given IM diclofenac 1.5mg/kg when VAS>3 and the time at which the analgesic was given was recorded, and the duration of analgesia was noted as time from deflation of tourniquet up to injection of diclofenac. Hypotension defined as a 25% decrease from baseline and was treated with IV ephedrine 3-6mg bolus. Bradycardia, defined as a 25% decrease from baseline was treated with IV Atropine 0.5mg. Arterial oxygen saturation less than 91% on room air was treated with 02 supplemented via face mask. During the study period any other complications such as dry mouth, nausea/vomiting or respiratory depression were noted.
Using data from a previous study by Memis et al., considering an alpha level of 0.05 and beta level of 0.90 to establish a desired power of 95% using two-sample means test assuming unequal variance, at least 30 patients were required in each group [10]. The data was analysed using the software SPSS 14 for windows. Student t-test was used for evaluation of the demographic data, intra-operative and post-operative haemodynamic variables, the onset and recovery times of sensory and motor block, duration of analgesia and intra-operative analgesic consumption and tourniquet pain. Friedman’s test was used for intra-operative and post-operative VAS and sedation scores. Level of significance was determined at p >0.05 for not statistically significant and p <0.05 for statistical significance.
Results
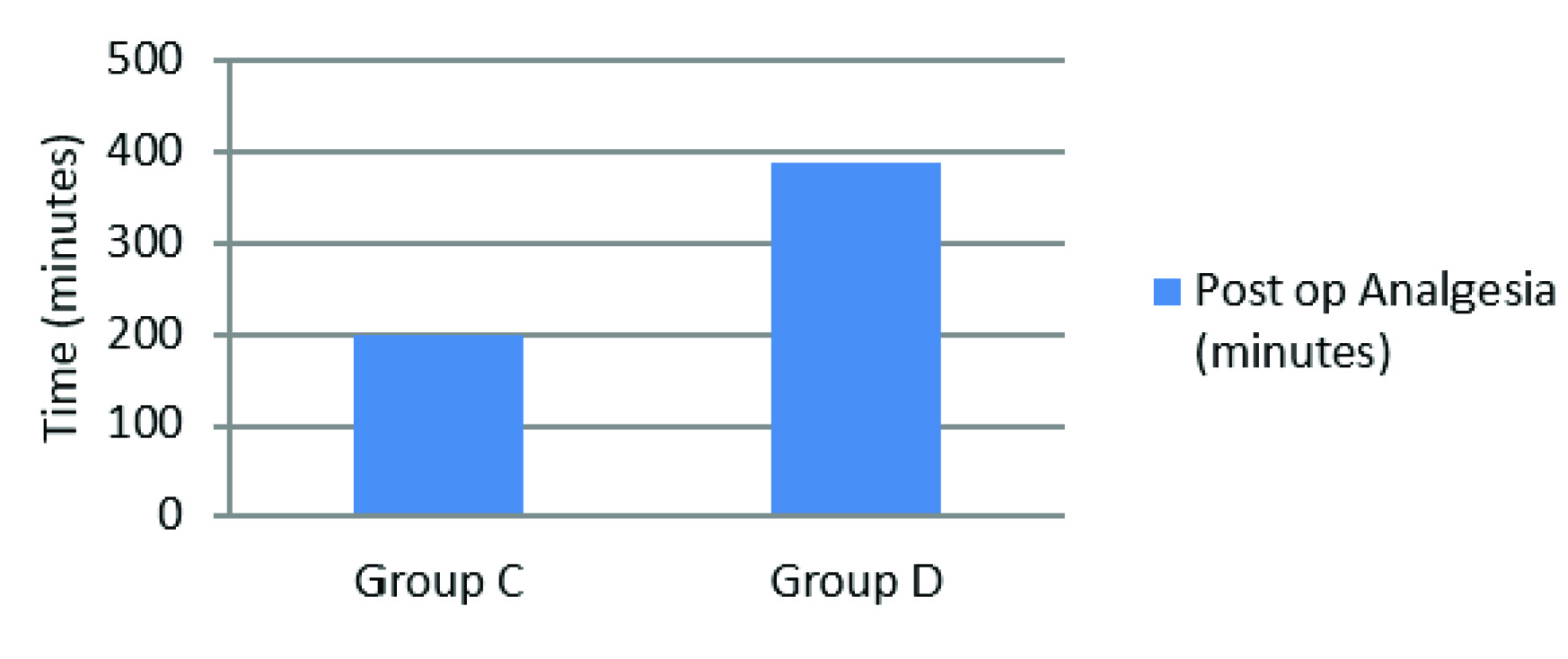
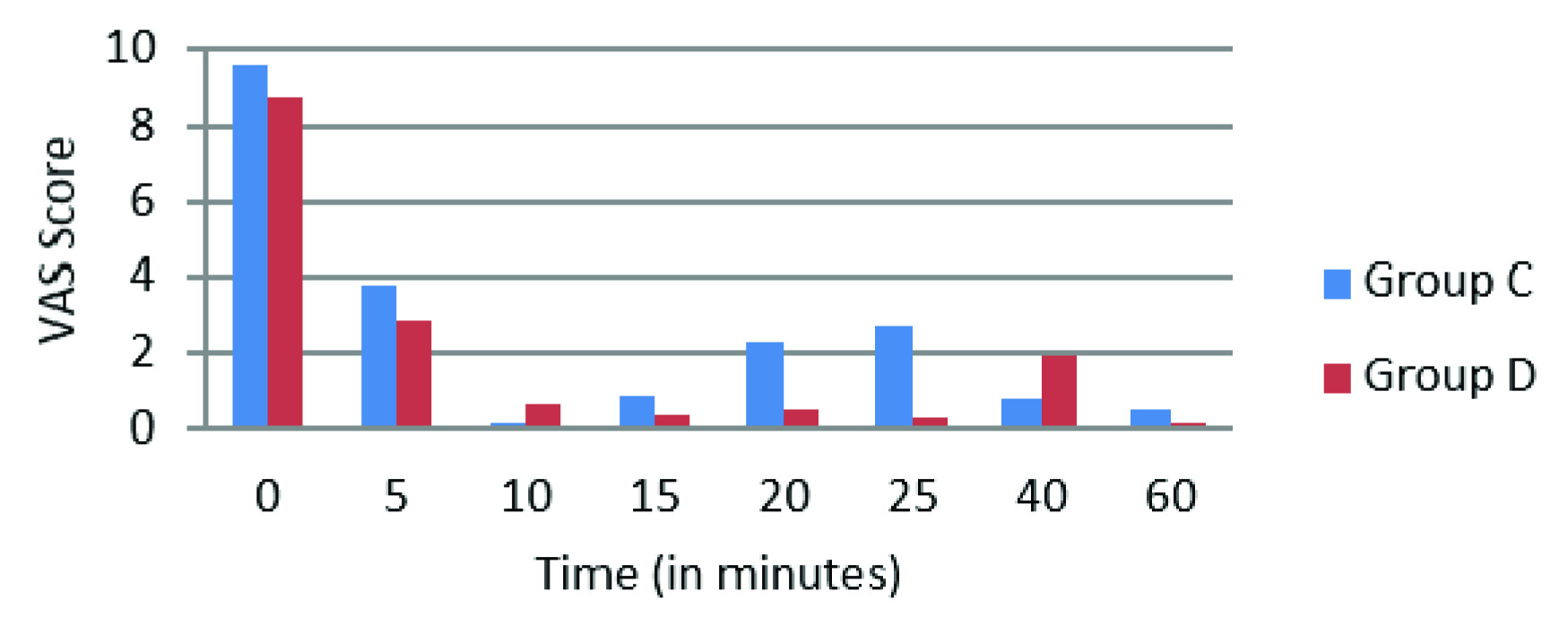
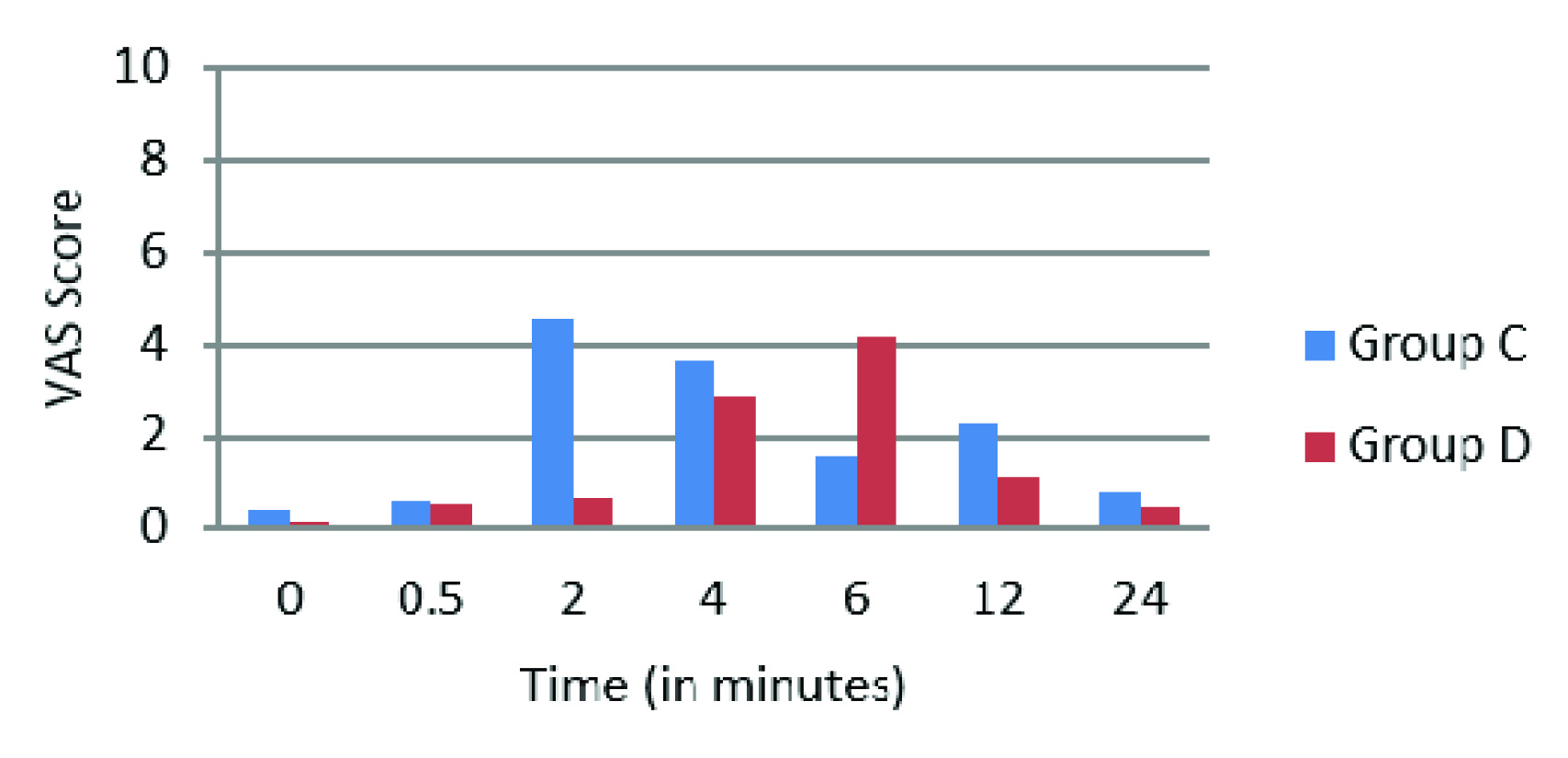
A total of 60 patients were enrolled in the study. The group C was control and group D was Dexmedetomidine (n=30 in each group), both groups were found comparable in terms of age, gender, ASA grading, weight, types of fractures and surgeries (p-values>0.05) as seen in [Table/Fig-1]. The onset of sensory and motor block was faster in group D as compared to group C (Sensory Block onset p-value-0.001, Motor Block onset p-value-0.034), similarly prolonged recovery times were noted for both sensory and motor block in group D (Sensory Block Recovery p-value-0.007, Motor Block Recovery p-value-0.001) (Sensory block-[Table/Fig-2] and Motor block-[Table/Fig-3]). There was no case of failure of block hence, general anaesthesia was not administered to any patient. The duration of post-operative analgesia was found to be longer in group D 388.53±104.71 minutes as compared to group C 200.5 ± 55.86 minutes (p-value- 0.009) [Table/Fig-4], in addition the incidence of tourniquet pain was less frequent. Eight patients in dexmedetomidine group complained of tourniquet pain as compared to 19 patients in control group. The mean onset of tourniquet pain was also noted to be delayed in dexmedetomidine group 43.75±6.944 min as compared to 36.63±7.266 min in control. The intra-operative fentanyl consumption was found to be 35.30±27.797μgs in group C as compared to 16.00±27.241μgs in group D (p-value-0.009). In group C the VAS scores>3 were noted from 2 to 4 hours after tourniquet deflation whereas in group D the VAS scores>3 were noted at 6 hours. After 6 hours upto the end of the study at 24 hours, lower VAS scores were noted in group D compared to group C (p-value <0.05) [Table/Fig-5,6].
Patient characteristics and outcomes.
| Parameters | Group C (n=30) | Group D(n=30) | p-value |
|---|
| Age in years(Mean ± SD) | 33.97 ±11.23 | 34.9 ± 11.95 | >0.05 |
| Gender : Male/Female | 17/13 | 20/10 | >0.05 |
| Weight in Kgs(Mean ± SD) | 55.7 ± 6.215 | 57.73 ± 6.721 | >0.05 |
| ASA Grades(1/2) | 29/1 | 27/3 | >0.05 |
| Duration of Surgery in minutes (Mean ± SD) | 49.17±12.183 | 52.33±9.803 | >0.05 |
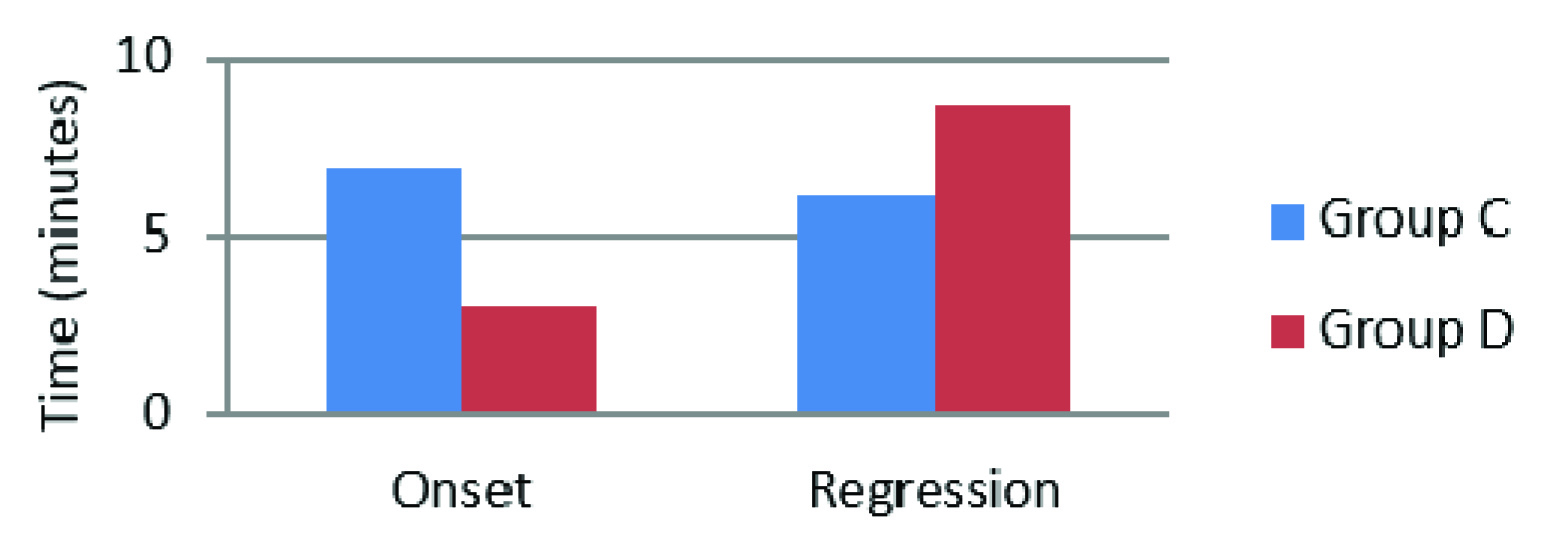
| Sensory Block onset (minutes) | 6.98 ± 3.4 | 3.02 ± 1.411 | 0.001 |
| Sensory Block Regression (minutes) | 6.20 ± 3.134 | 8.63 ± 3.783 | 0.007 |
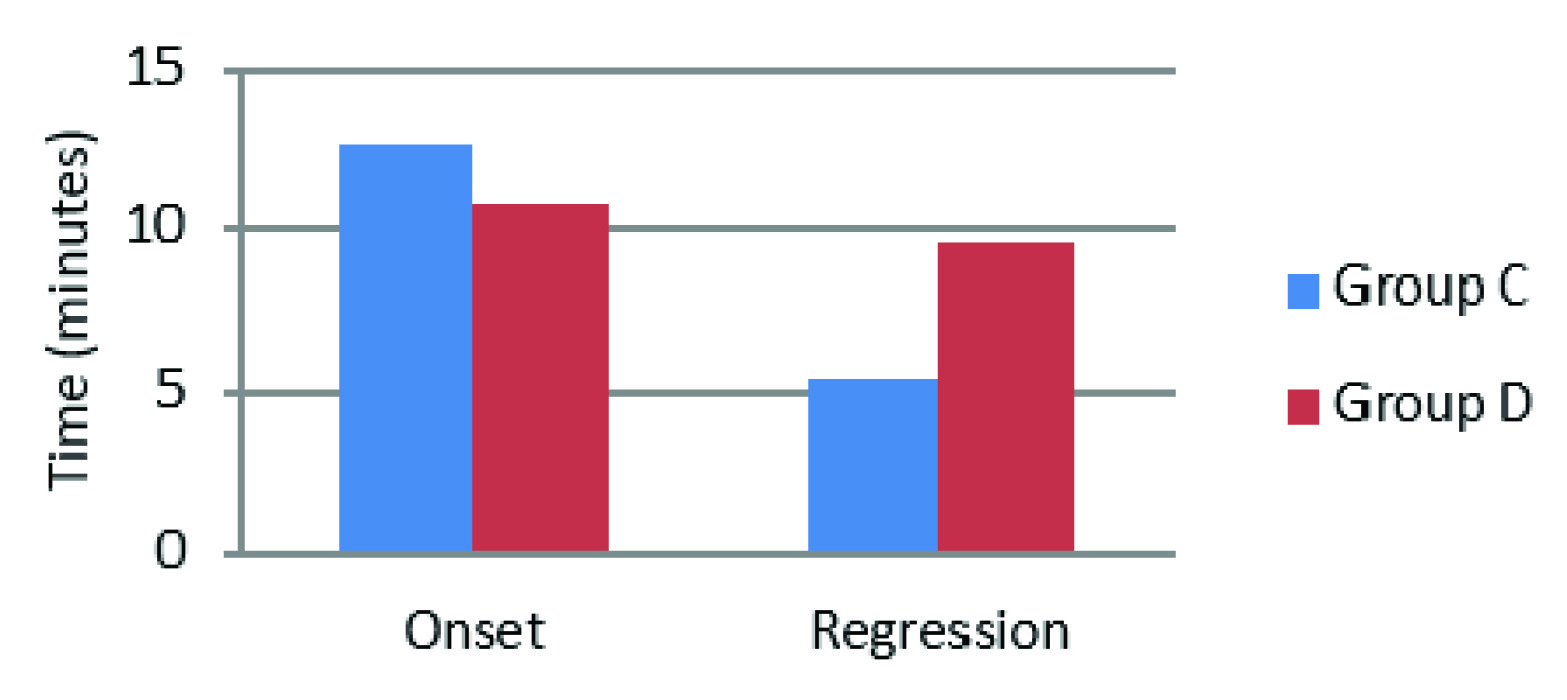
| Motor Block onset (minutes) | 12.67 ± 3.188 | 10.73 ± 3.676 | 0.034 |
| Motor Block Regression (minutes) | 5.4 ± 2.541 | 9.6 ± 5.775 | 0.001 |
| Post-op Analgesia (minutes) | 200.5 ± 55.86 | 388.53±104.71 | 0.009 |
| Tourniquet Pain onset (minutes) | 36.63±7.266 | 43.75±6.944 | 0.027 |
| Tourniquet Pain incidence | 19/30 | 8/30 | - |
| Intra-op fentanyl (μgs) | 35.30±27.797 | 16.00±27.241 | 0.009 |
Onset and regression of sensory block
Onset and regression of motor block
Duration of post-operative analgesia
Intra-operative VAS scores
Post-operative VAS scores
The heart rates in both groups remained comparable throughout the intra-operative period. Though bradycardia was noted in 3 patients of group D at 40 minutes after tourniquet inflation, it was very transient and recovered before atropine could be administered. No episode of bradycardia was noted post-operatively in either group. The mean arterial pressures, and sedation scores were found to be comparable in both groups; no episode of hypotension was noted in either group during or after surgery.
Discussion
IVRA provides safe, rapid effective analgesia with muscle relaxation for patients undergoing extremity surgery when the surgical procedure is expected to last for less than an hour [1]. It was first described by August Bier in 1908, his technique involving the use of 50 to 100mL 0.5 % procaine solution [12–14].
IVRA has been used in short outpatient procedures in the emergency department and for complex regional pain syndromes [1,15]. Its limited use has been mainly due to the short duration of the block, occurrence of tourniquet pain and no analgesic effect after the tourniquet is released. But various side effects such as delayed respiratory depression, pruritis nausea and very short duration of analgesia after release of tourniquet have necessitated the continued study of newer additives to the block. Adrenergic agonists have been used in regional anaesthesia because of their anxiolytic, sedative, perioperative sympatholytic, cardiovascular stabilizing properties and decreased anaesthetic requirements [7–9]. Clonidine α2 agonist has shown improved tourniquet tolerance and effective post-operative analgesia as an additive to Biers block in a study by Gentili et al., [16]. However, in a study by Kleinschmidt et al., there was no significant post-operative analgesia or change in onset and regression of block [17]. Several other studies using clonidine showed a lack of dose-responsiveness for benefit and risk, furthermore hypotension and post-tourniquet release sedation decreased its use [3,18].
Dexmedetomidine has α2:α1ratio of 1600:1 making it a highly selective α2 agonist compared to clonidine (α2:α1ratio of 200:1) [9], this reduces the unwanted side effects due to α1 receptors such as hypotension [19]. Higher selectivity of dexmedetomidine to α2A receptors (which mediate analgesia and sedation) have been stated in regional anaesthesia practice [19]. In a systematic review article by Guay J., the addition of dexmedetomidine in block provided protection from local anaesthetic-induced central nervous system and cardiac toxicity with IVRA [20], fewer side effects and lesser sedation unlike clonidine. Dexmedetomidine is more lipophilic than clonidine (about 3.5 times), thus prolonging the duration of sensory and motor blockade induced by local anaesthetics, irrespective of the route of administration [8]. In a study by Yoshitomi et al., the local anaesthetic action of lignocaine was documented to be enhanced with the addition of dexmedetomidine via α2 adrenoceptors [21].
The selected dose of dexmedetomidine of 0.5μg/kg was based on a study by Memis et al., who detected longer post-operative analgesia, improved tourniquet tolerance and significantly reduced VAS scores without additional side effects [10]. Hence we performed this study to evaluate the efficacy of dexmedetomidine of 0.5μg/kg as an adjuvant to lignocaine for IVRA.
The two study groups were found comparable in demographic characteristics (age, weight, ASA grades, type of fractures and surgical procedures and the duration of surgery), and these findings were similar to those in the study by Memis et al., [10]. The onset of sensory block in Dexmedetomidine group) was significantly shorter and the regression of sensory block was significantly longer than that of the control group [Table/Fig-1]. These findings were similar to the study by Memis et al., where shortened onset of sensory block and prolonged regression with dexmedetomidine were reported [10]. In the study by Kol IO et al., a longer duration of sensory block was similarly noted when dexmedetomidine 0.5μg /kg was added to prilocaine in IVRA [2].
Similarly the onset of motor block was faster and regression of motor block was slower which were found statistically significant [Table/Fig-1]. This was in concurrence with the studies by Kol IO et al., Memis et al., and Mizrak et al., where the addition of dexmedetomidine 0.5μg /kg to IVRA had shortened motor onset and prolonged recovery of the motor block [2,10,11].
In the present study, we recorded the time for demand of rescue analgesic as a measure of post-operative analgesia. Significantly longer duration of post-operative analgesia was also noted in the dexmedetomidine group. Memis et al., had similar findings in dexmedetomidine group [10]. The study by Kol IO et al., also showed prolonged post-operative analgesia in the dexmedetomidine group when added to prilocaine [2]. In the present study overall post-operative VAS scores in the dexmedetomidine group were found lower than that of control. VAS scores of more than three requiring IM diclofenac were found at four to six hours after surgery in dexmedetomidine group unlike control group which was noted earlier after two to four hours. Significantly lower VAS scores in the dexmedetomidine group were also seen in studies by Kol IO et al., and Memis et al., [2,10].
Because of the rapid reperfusion of the limb after tourniquet deflation, IVRA using local anaesthetic solution without any adjuvants typically would provide minimal post-operative analgesia. Intravenously administered dexmedetomidine has been shown to produce analgesic effects by acting at both spinal and supraspinal levels, the analgesic effect primarily is mediated from inhibition of locus ceruleum at brainstem and is also due to pre-synaptic activation of α2 receptors inhibiting norepinephrine release thereby terminating the propagation of pain signals and post-synaptically inhibiting sympathetic activity making the patient pain free, comfortable for a longer duration post-surgery compared to lignocaine alone [22].
It was also noted that the mean fentanyl consumption intra-operatively was reduced in the dexmedetomidine group. In the study by Memis et al., the intra-operative fentanyl consumption was also higher in the control group, which was also similar to our study [10].
Tourniquet pain is a traditional limitation of IVRA which, manifests as a dull and aching sensation at site of tourniquet application, which increases in severity with duration of inflation, which only resolves with deflation of the tourniquet. Theories regarding aetiology of the pain suggest nerve ischaemia and compression as the main causes [23]. Unmyelinated C fibres (not active before tourniquet inflation and not affected by movement or anaesthesia) have been recognized as representing the major pathway of pain, since these fibres get continuously stimulated by skin compression, also as C fibres are more resistant to local anaesthetic conduction block. The incidence of tourniquet pain differed significantly with the control group having more incidences in the present study. Similar findings of improved tourniquet tolerance were also seen with studies by Kol IO et al., Memis et al., and Mizrak et al., with dexmedetomidine at a dose of 0.5μg /kg [2,10,11].
Dexmedetomidine could result in cardiovascular depression, i.e., bradycardia and hypotension [24], this cardiovascular response is mainly due to reduced sympathetic outflow. The incidence of post-operative bradycardia has been reported as high as 40% in healthy surgical patients who received dexmedetomidine as sedation, especially in doses of more than 1μg/kg. Usually, these temporary effects were successfully treated with atropine or ephedrine and volume infusions [25]. In a systematic review of nine randomized controlled trials studying perineal dexmedetomidine added to brachial plexus blocks, 7% of patients developed reversible bradycardia, but no effect was noted on the incidence of hypotension [26]. Jaakola ML., analysed dexmedetomidine as a pre-medicant prior to IVRA and noted a 20% decrease in systolic as well as in diastolic pressure and heart rate which was statistically significant when compared with the control group which returned to pre-operative values in 4 hours [27]. In both groups, in the present study, the heart rates and blood pressure remained comparable throughout the study period. Though bradycardia was noted transiently in three patients of group D at 40 minutes after tourniquet inflation, it recovered spontaneously without atropine.
Sedation scores were comparable in both groups, which were found concurrent with the study by Memis et al., with the dose of dexmedetomidine as 0.5μg/kg [10]. Side effects such as dry mouth, nausea/vomiting or respiratory depression were not noted in patients of either group, these findings were concurrent with earlier studies [10,11]. Hence, we conclude that an intravenous dose of 0.5μg/kg of dexmedetomidine does not lead to a decrease in saturation or respiratory depression even when used as an additive to IVRA.
Limitation
Biers blocks or IVRA is a technique which can be administered in both upper and lower limbs, restricting our study to only the upper limbs would have been one limitation. A second limitation could have been using a confidence interval of 95% which was similar to the parent study; probably increasing the confidence interval to 98% with a larger number of subjects could have enhanced the sensitivity of our study.
Conclusion
In conclusion this study demonstrated that the addition of dexmedetomidine at 0.5μg /kg to a 40ml 0.5% solution of lignocaine in IVRA improved the quality of block, both sensory and motor block. It decreased the incidence of tourniquet pain, enhanced the duration of post-operative analgesia and was not associated with haemodynamic instability or any other complications.