Expression of Alpha Methylacyl CoA Racemase (AMACR) in Gastric Adenocarcinoma and Its Correlation with Helicobacter pylori Infection
Yamini Jindal1, Anshul Singh2, Ravikant Kumar3, Kachnar Varma4, Vatsala Misra5, Sri Prakash Misra6, Manisha Dwivedi7
1 Junior Resident, Department of Pathology, M.L.N. Medical College, Allahabad, U.P., India.
2 Assistant Professor, Department Pathology, M.L.N. Medical College, Allahabad, U.P., India.
3 Senior Resident, Department of Gastroenterology M.L.N. Medical College, Allahabad, U.P., India.
4 Associate Professor, Department of Pathology, M.L.N. Medical College, Allahabad, U.P., India.
5 Professor and Head, Department of Pathology, M.L.N. Medical College, Allahabad, U.P., India.
6 Professor and Head, Department of Gastrology, M.L.N. Medical College, Allahabad, U.P., India.
7 Professor, Department of Gastrology, M.L.N. Medical College, Allahabad, U.P., India.
NAME, ADDRESS, E-MAIL ID OF THE CORRESPONDING AUTHOR: Dr. Yamini Jindal, Junior Resident, Department of Pathology, M.L.N. Medical College, Allahabad-211001, U.P., India.
E-mail: yaminijindal5@gmail.com
Introduction
Gastric cancer develops in a multistep progression and is determined by genetic and environmental factors. Over-expression of Alpha Methylacyl CoA Racemase (AMACR) is useful in diagnosis of prostate cancer. There is plenty of genetic alteration that occurs in gastric adenocarcinoma. The present study was planned to determine if AMACR can be used as a diagnostic marker in gastric adenocarcinoma similar to prostate cancer.
Aim
To study the expression of AMACR in gastric adenocarcinoma and correlate its expression with density of Helicobacter pylori.
Materials and Methods
This cross-sectional, prospective study was conducted from August 2013-2015. Fifty gastric cancer biopsies were taken. Adjacent biopsy from normal/reactive mucosa was also taken from 21 cases. Samples were stained with H&E for morphological details, Loeffler’s methylene blue for Helicobacter pylori and immunohistochemistry (IHC) was done to check for the expression of AMACR proteins.
Statistical analysis was done using chi square test, Spearman’s correlation coefficient and Fisher’s exact test. The p-value ≤ 0.05 was taken as critical level of significance.
Results
Overexpression of AMACR was observed in 88.89% of intestinal type and 78.05% of diffuse type adenocarcinoma. AMACR expression was significantly less in adjacent reactive/dysplastic mucosa. Helicobacter pylori were seen in 8/9 (88.89%) and 35/41(85.36%) cases of intestinal adenocarcinoma and diffuse adenocarcinoma respectively. When grades of Helicobacter pylori were compared with the positivity of AMACR, no significant association and correlation was found.
Conclusion
The expression of AMACR in neoplastic tissue was significantly higher as compared to adjacent dysplastic, reactive or normal tissue. Thus, IHC for AMACR can be used for differentiating the cases of reactive atypia from early neoplastic lesions similar to its role in prostatic tissue. Helicobacter pylori does not affect the expression of AMACR in neoplastic gastric lesions.
Diffuse type adenocarcinoma, Immunohistochemistry, Intestinal type adenocarcinoma
Introduction
Among cancers, stomach cancer is the fifth leading cancer and the third leading cause of death from cancer making up 7% of cases and 9% of deaths (World Cancer Report 2014). In India, gastric cancer is ranked as the fifth and seventh most common cancer in males and females, respectively [1] and is the second leading cause of death from cancer in both men and women [2].
Gastric cancer develops in a multistep progression of precancerous lesions and is primarily determined by environmental factors [3]. Plenty of risk factors like old age [4], non-vegetarian and high salty pickled diet [5], tobacco [6] and alcohol usage [7], radiation [8] and family history [9] are responsible for the genesis of gastric cancer.
Helicobacter pylori, previously named Campylobacter pylori, is a helix-shaped gram-negative, bacterium found in the stomach. Prevalence of Helicobacter pylori has been described in precancerous lesions [10] and is classified as a group I carcinogen, especially of intestinal type gastric carcinoma [11].
However, over 80% of individuals infected with the bacterium are asymptomatic and it may play an important role in the natural stomach ecology [12]. The host’s genetic makeup, dietary and environmental factors might explain this mystery. Hence, studies on H. pylori in relation to genetic and environmental risk factors are required.
AMACR, (α Methylacyl coenzyme A racemase also known as P504S), is a mitochondrial and peroxisomal enzyme that plays a role in the beta-oxidation of fatty acid derivatives by catalyzing interconversion of several (2R)-methyl branched chain fatty acyl-coenzyme A esters to their (S) stereoisomers [13].
This protein was originally identified in mitochondria and peroxisomes of rat liver cells [14] and was then isolated by cDNA subtraction and microarray analysis. Over-expression of AMACR is reported to be a useful marker in diagnosis of prostate cancer because its expression is increased in premalignant and malignant lesions and it helps to differentiate between reactive atypia from true neoplastic changes [15,16]. So the present study was planned to evaluate the expression of AMACR in gastric adenocarcinoma cases along with adjacent normal/reactive mucosa and correlate its expression with density of Helicobacter pylori.
Materials and Methods
Prospective study of two years was done (from August 2013- August 2015) and 50 gastric cancer samples were taken. Adjacent biopsy from endoscopically normal/reactive mucosa was also taken from 21 cases. Samples were stained with:
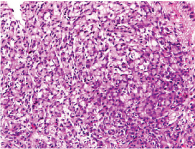
Haematoxylin and Eosin for morphological details. Intestinal type [Table/Fig-1] and diffuse type [Table/Fig-2] adenocarcinoma.
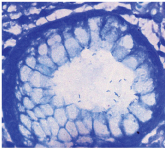
Loeffler’s methylene blue- for Helicobacter pylori [Table/Fig-3].
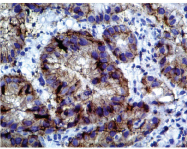
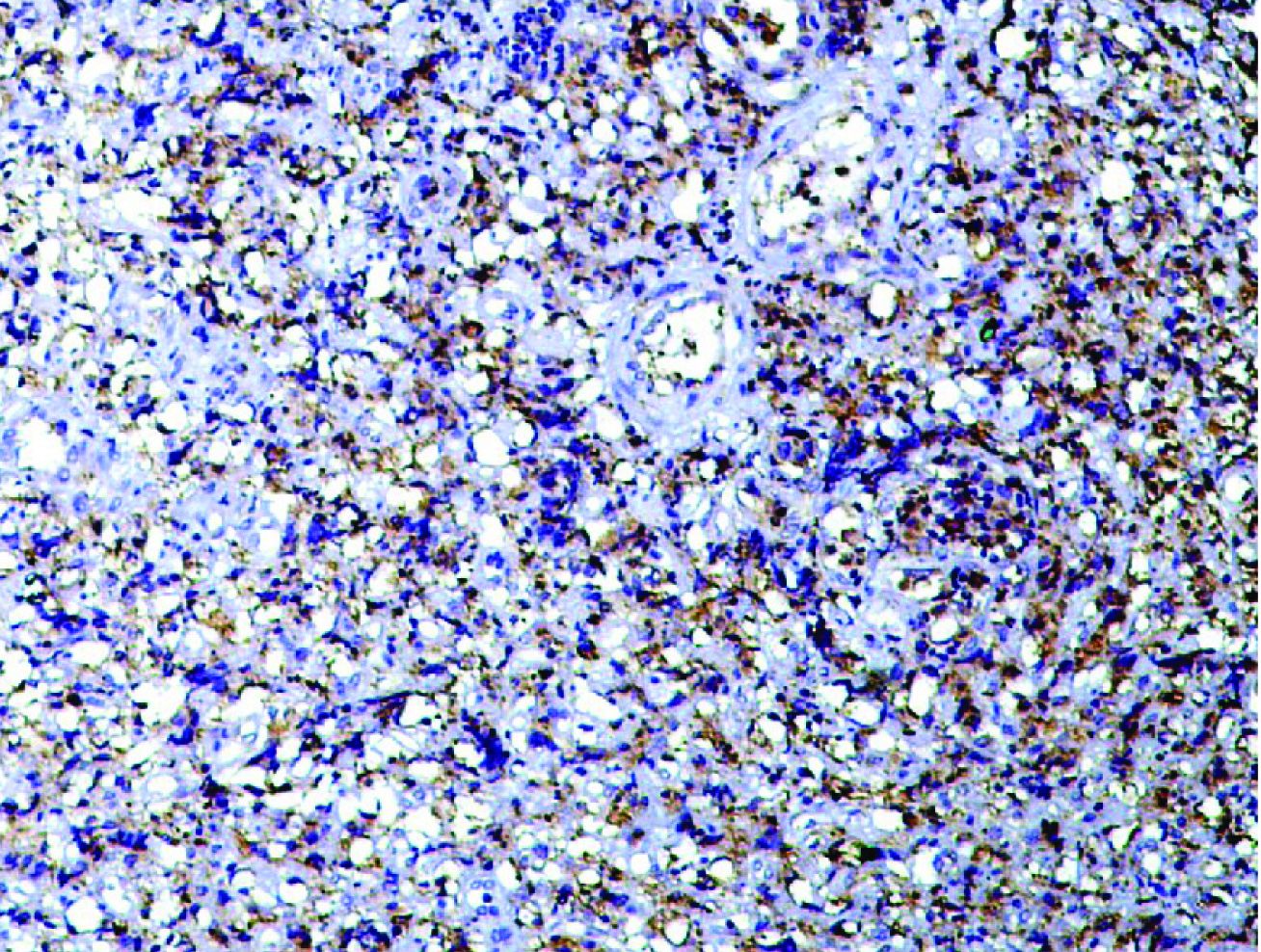
Immunohistochemistry for the expression of AMACR proteins in Intestinal type [Table/Fig-4] and diffuse type [Table/Fig-5] adenocarcinoma. AMACR expression was scored according to Huang et al., [17].
Intestinal type adenocarcinoma with intestinal metaplasia in adjacent pit (H&E 400x).
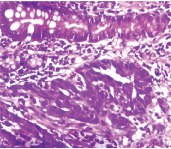
Diffuse type adenocarcinoma (H&E 100x).
Helicobacter pylori. (Loeffler’s methylene blue stain - 1000x).
AMACR expression in intestinal type gastric adenocarcinoma. (IHC 400x).
AMACR expression in diffuse type gastric adenocarcinoma. (IHC 100x).
Primary antibody used for AMACR: Monoclonal Rabbit Anti- P504S / AMACR clone RBT-AMACR (BioGenex, Fremont CA).
Statistical analysis was done using chi square test, Spearman’s correlation coefficient and Fisher’s exact test. The p-value ≤ 0.05 was taken as critical level of significance.
Results
Out of 50 total samples of adenocarcinoma, 9 were intestinal and 41 were diffuse carcinoma. Helicobacter pylori was seen in 8/9 (88.89%) and 35/41 (85.36%) of intestinal and diffuse carcinoma respectively and was graded [Table/Fig-6].
Grading of Helicobacter pylori.
| Helicobacter pylori | Intestinal Type n(%) | Diffuse Type n(%) |
|---|
| Grade 0 | 1 (11.1%) | 6 (14.63%) |
| Grade 1 | 1 (11.1%) | 4 (9.75%) |
| Grade 2 | 2 (22.2%) | 14 (34.15%) |
| Grade 3 | 5 (55.5%) | 17 (41.46%) |
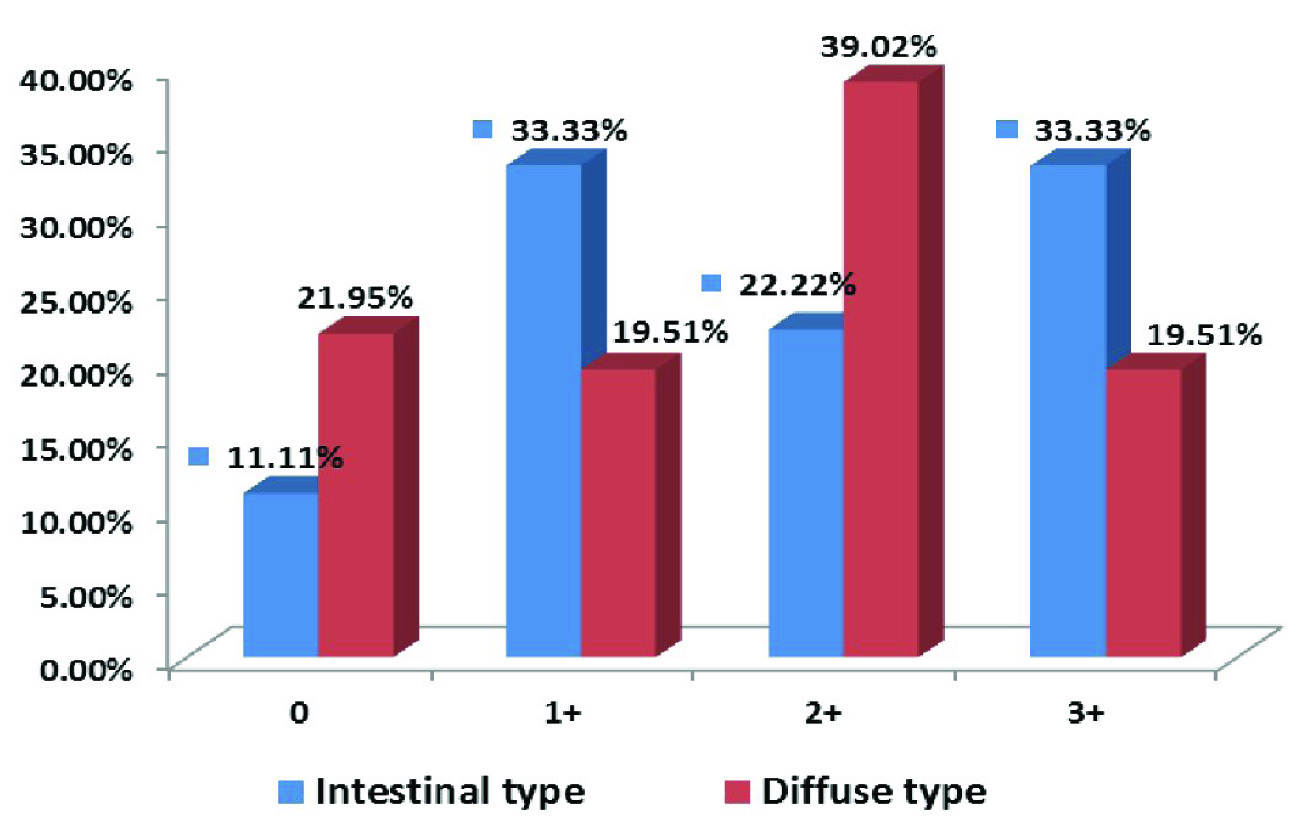
Sections which showed expression of AMACR in >5% tumour cell were considered true positive and this was seen in 40/50 cases of gastric adenocarcinoma {8/9 (88.89%) and 32/41 (78.05%) of intestinal and diffuse adenocarcinoma respectively}. They were further scored according to description by Huang et al., [Table/Fig-7] [17]. Difference between expression of AMACR in intestinal in 8/9 (88.89%) and diffuse type adenocarcinoma in 32/41 (78.05%) was compared using chi square test and it was found to be significant (p < 0.05).
Intensity of expression of AMACR in intestinal and diffuse type adenocarcinoma.
Intensity of AMACR was also scored in adjacent biopsies [Table/Fig-8]. AMACR expression was higher in neoplastic epithelium (40/50) than adjacent epithelium (11/21) and the difference between them was found to be statistically significant according to Fisher’s-exact test (p < 0.05).
Intensity of AMACR expression in adjacent biopsies.
| Score | Total Cases n(%) |
|---|
| 0 | 10 (47.62%) |
| 1+ | 7 (33.33%) |
| 2+ | 3 (14.29%) |
| 3+ | 1 (4.76%) |
When grades of Helicobacter pylori were compared with the positivity of AMACR according to Spearman’s correlation coefficient, no significant association and correlation was found [Table/Fig-9].
Correlation of Helicobacter pylori with AMACR in gastric cancer.
| Helicobacter pylori | AMACR (0) | AMACR (1+) | AMACR (2+) | AMACR (3+) |
|---|
| Grade 0/ Inconclusive | 2 (4%) | 2 (4%) | 2 (4%) | 1 (2%) |
| Grade 1 | 0 (0%) | 0 (0%) | 3 (6%) | 2 (4%) |
| Grade 2 | 3 (6%) | 2 (4%) | 8 (16%) | 3 (6%) |
| Grade 3 | 5 (10%) | 7 (14%) | 5 (10%) | 5 (10%) |
Discussion
Helicobacter pylori is a microorganism, whose infection has been defined in etiology of gastric cancer development. Concomitance of Helicobacter pylori and gastric cancer has been reported at different rates from different studies. Studies in Asian countries such as Thailand, India, Bangladesh, Pakistan, Iran, Saudi Arabian countries, Israel and Malaysia, have reported a high frequency of Helicobacter pylori infection co-existing with a low incidence of gastric cancer [18]. The prevalence of Helicobacter pylori was reported to be more in controls than in patients group (80% vs 78%) [19].
In earlier study from this center, prevalence of Helicobacter pylori was more in diffuse type of gastric cancer than intestinal type (86% vs 68%) [19], whereas studies showed high prevalence in intestinal type (89.2%) than in diffuse type (31.8%) adenocarcinoma [20]. Quigley et al., in their review stated that human epidemiological studies have produced mixed results with an association between Helicobacter pylori and gastric cancer in 50% patients, while the remaining patients showed a negative relationship [21].
In the present study, Helicobacter pylori were seen in 88.89 % and 78.05% in intestinal and diffuse adenocarcinoma respectively. However, difference was not statistically significant and that was probably due to small number of intestinal type adenocarcinoma studied when compared according to grades of Helicobacter pylori positivity, no difference was observed in two groups.
In the present study, true positive cases for AMACR expression were 88.89% and 78.05% cases of intestinal and diffuse type adenocarcinoma respectively. Expression was significantly higher in intestinal type as compared to diffuse type adenocarcinoma. Intensity of AMACR expression in adjacent biopsies was also seen. AMACR expression was higher in neoplastic epithelium than adjacent epithelium and the difference between them was found to be statistically significant.
Huang et al., found that AMACR was not expressed in the gastric mucosal specimens with negative and indefinite for dysplasia, but it was observed in 40.8% gastric biopsy specimens with dysplasia [17].
However, AMACR was more common in intestinal type adenocarcinoma than diffuse type. But expression was significantly less in adjacent normal mucosa. These findings suggest that AMACR immunoreactivity was fairly sensitive in identification of high-grade dysplasia and is highly specific in distinguishing high-grade dysplasia from reactive atypia. Thus, expression of AMACR can be taken as indicator of neoplastic change in gastric adenocarcinoma similar to prostate neoplasia and its expression increases from preneoplastic to neoplastic lesion.
When grades of Helicobacter pylori were compared with the positivity of AMACR, no significant association and correlation was found. That was similar to findings found by Huang et al., [17].
Conclusion
The expression of AMACR in neoplastic tissue was significantly higher as compared to adjacent dysplastic, reactive or normal tissue. Thus, highlighting that IHC for AMACR can be used for differentiating the cases of reactive atypia from early neoplastic lesions similar to its role in prostatic tissue. Helicobacter pylori does not affect the expression of AMACR in neoplastic gastric lesions.
[1]. Brenner H, Rothenbacher D, Arndt V, Epidemiology of stomach cancerMethods Mol Biol 2009 472:467-77. [Google Scholar]
[2]. Dikshit R, Gupta PC, Ramasundarahettige C, Gajalakshmi V, Aleksandrowicz L, Rajendra Badwe R, Cancer mortality in India: a nationally representative surveyThe Lancet 2012 379:1807-16. [Google Scholar]
[3]. You WC, Li JY, Zhang L, Jin ML, Chang YS, Ma JL, Etiology and prevention of gastric cancer: a population study in a high risk area of ChinaChin J Dig Dis 2005 6(4):149-54. [Google Scholar]
[4]. Lazar D, Taban S, Dema A, Cornianu M, Goldis A, Ratiu I, Gastric cancer: the correlation between the clinicopathological factors and patients’ survival (I)Rom J Morphol Embryol 2009 50(1):41-50. [Google Scholar]
[5]. Ren JS, Kamangar F, Forman D, Islami F, Pickled Food and Risk of Gastric Cancer – a Systematic Review and Meta-analysis of English and Chinese LiteratureCancer Epidemiol Biomarkers Prv 2012 21(6):905-15. [Google Scholar]
[6]. La Torre G, Chiaradia G, Gianfagna F, De Lauretis A, Boccia S, Mannocci A, Smoking status and gastric cancer risk: an updated meta-analysis of case-control studies published in the past ten yearsTumouri 2009 95:13-22. [Google Scholar]
[7]. Tramacere I, Pelucchi C, Bagnardi V, Rota M, Scotti L, Islami F, A meta-analysis on alcohol drinking and gastric cancer riskAnn Oncol 2012 23:28-36. [Google Scholar]
[8]. Cogliano VJ, Baan R, Straif K, Preventable exposures associated with human cancersJNCI 2011 103:1827-39. [Google Scholar]
[9]. Yaghoobi M, Bijarchi R, Narod SA, Family history and the risk of gastric cancerBJC 2010 102:237-42. [Google Scholar]
[10]. Lyon. Schistosomes, liver flukes and Helicobacter pylori. IARC Working Group on the Evaluation of Carcinogenic Risks to HumansIARC Monogr Eval Carcinog Risks Hum 1994 61:1-241. [Google Scholar]
[11]. Neubauer A, Thiede C, Alpen B, Ritter M, Neubauer B, Ehninger G, Cure of Helicobacter pylori infection and duration of remission of low grade gastric mucosa associated lymphoid tissue lymphomaJ Natl Cancer Inst 1997 89:1350-55. [Google Scholar]
[12]. Blaser MJ, Who are we? Indigenous microbes and the ecology of human diseasesEMBO Reports 2006 7:956-60. [Google Scholar]
[13]. Went PT, Sauter G, Oberholzer M, Bubendorf L, Abundant expression of AMACR in many distinct tumour typesPathology 2006 38:426-32. [Google Scholar]
[14]. Ferdinandusse S, Denis S, Clayton PT, Graham A, Rees JE, Allen JT, Mutations in the gene encoding peroxisomal AMACR cause adult-onset sensory motor neuropathyNat Genet 2000 24:188-91. [Google Scholar]
[15]. Wiklund F, Gillanders EM, Albertus JA, Bergh A, Damber JE, Emanuelsson M, Genome-wide scan of Swedish families with hereditary prostate cancer: Suggestive evidence of linkage at 5q11.2 and 19p13.3Prostate 2003 57:290-97. [Google Scholar]
[16]. Jiang Z, Wu CL, Woda BA, Alpha-methylacyl-CoA racemase: a multi-institutional study of a new prostate cancer markerHistopathology 2004 45:218-25. [Google Scholar]
[17]. Huang W, Zhao J, Li Li, Huang Y, Yang X, Wang J, α-Methylacyl coenzyme A racemase is highly expressed in the intestinal-type adenocarcinoma and high-grade dysplasia lesions of the stomachHistopathol 2008 23:1315-20. [Google Scholar]
[18]. Goh KL, Epidemiology of Helicobacter pylori infection in Malaysia—observations in a multiracial Asian populationMed J Malaysia 2009 64:187-92. [Google Scholar]
[19]. Misra V, Misra SP, Singh MK, Singh PA, Dwivedi M, Prevalence of Helicobacter pylori in patients with gastric cancerIndian J Pathol Microbiol 2007 50:702-07. [Google Scholar]
[20]. Parkin DM, Cancers attributable to infection in the UK in 2010BJC 2011 105:49-56. [Google Scholar]
[21]. Quigley EM, Keohane J, DyspepsiaCurrent Opinion Gastroenterology 2008 24:692-97. [Google Scholar]