In women of all age groups ovarian masses are a common occurrence, with approximately 8% of asymptomatic women aged between 25 to 40 years. About 80% of ovarian tumours are benign, and these occur mostly in young women between 20 and 45 years whereas borderline tumours occur at slightly older age. Incidence of malignant tumours increases with age, occurring predominantly in pre-menopausal and perimenopausal women. Ovarian cancer is the sixth most common cancer among women and is also the seventh leading cause of cancer deaths among women worldwide [1,2]. Ovaries are the third leading site of cancer among women trailing behind cervical and breast cancer according to the Indian cancer registries [1,3].
90% of all ovarian carcinomas and two thirds of all ovarian neoplasms are surface epithelial tumours. These tumours assume a wide array of histological pattern making it an interesting topic for study. Knowledge of the type of tumour and differentiation helps in judicious management of the patient in terms of appropriate treatment and follow-up [4–6]. The aim of this study was to categorise these lesions into benign, borderline and malignant, to study their clinical and histopathological pattern and to compare their incidences with other studies.
Materials and Methods
This is a 5 year (3 years of retrospective and 2 years of prospective) study of 139 cases conducted over the period from June 2006 to May 2011 after obtaining Institutional Ethical Committee clearance. The ovarian tissue for the study was obtained from hysterectomy with unilateral or bilateral oophorectomy specimens, oophorectomy and ovarian cystectomy specimens that were received in the histopathology section of our department. The relevant clinical details about the patient were retrieved from the hospital data. All the lesions, which on histopathological examination, were diagnosed as surface epithelial tumours listed under the WHO classification of primary ovarian tumours were included in the study. Non-neoplastic lesions, tumour like conditions, paraovarian lesions and metastatic tumours were excluded. All the received samples were fixed in 10% buffered formalin. Careful gross examination was done including external appearance, capsule breech and examination of serial cut-sections. Cyst walls, solid and papillary areas and any unusual areas were sampled for microscopy. They were processed routinely; 4-5 micron thick sections were taken, stained with Haematoxylin and Eosin (H&E)and examined microscopically. The tumours were classified into benign, borderline and malignant categories based on the WHO classification [4]. Immunohistochemistry was done in cases of mucinous cystadenocarcinoma to prove that they were primary ovarian carcinoma and to exclude metastatic tumours [5,6].
Results
A total of 141 surface epithelial tumours of the ovary were seen in 139 patients with 2 patients having double pathology. Of the 141 tumours, 117 (83.01%) were benign, 7 (4.9%) were borderline and 17 (12.1%) were malignant [Table/Fig-1]. Serous cystadenoma (56, 39.7%) was the most common benign lesion [Table/Fig-2] followed by mucinous cystadenoma (46, 32.6%) [Table/Fig-3]. Out of the total malignant cases (17,12.1%), serous cystadenocarcinoma was most common (13, 9.22%) [Table/Fig-4], followed by 2 cases of mucinous cystadenocarcinoma [Table/Fig-5] and one each of clear cell carcinoma [Table/Fig-6] and endometrioid carcinoma [Table/Fig-6]. Out of the 7 cases of borderline lesions [Table/Fig-7] 4 were mucinous followed by 3 of the serous type. Age range varied from 14-76 years. Mean age at diagnosis was 42.4 years. Maximum number of patients were in the 40-49 year age group (35.3%). The most common clinical presentation was mass abdomen seen in 58.9% cases [Table/Fig-8]. The details regarding external surface, locularity and frequency of papillary projections have been mentioned in [Table/Fig-9].
Classification and distribution of surface epithelial tumours.
| Nature of Tumours | No. of Cases | Percentage |
|---|
| SEROUS TUMOURS | 87 | 61.7% |
| A. Benign | 71 | 50.35% |
| Serous cystadenoma | 53 | 37.5% |
| Papillary serous cystadenoma | 8 | 5.6% |
| Serous cystadenofibroma | 6 | 4.2% |
| Adenofibroma | 2 | 1.4% |
| Serous paillary cystadenofibroma | 2 | 1.4% |
| B. Borderline | 3 | 2.1% |
| Serous | 1 | 0.7% |
| Papillary serous | 1 | 0.7% |
| Serous cystadenofibroma | 1 | 0.7% |
| C. Malignant | 13 | 9.2% |
| Serous cystadeno carcinoma | 5 | 3.5% |
| Papillary serous cystadeno carcinoma | 7 | 4.9% |
| Solid type | 1 | 0.7% |
| Seromucinous Cystadenoma | 3 | 2.1% |
| Mucinous | 46 | 32.6% |
| A. Benign | 40 | 28.6% |
| Cystadenoma | 39 | 27.9% |
| Cystadenofibroma | 1 | 0.7% |
| B. Borderline | 4 | 2.8% |
| C. Malignant | 2 | 1.4% |
| Cystadenocarcinoma | 2 | 1.4% |
| Transitional - Brenner Tumour | 3 | 2.1% |
| Clear Cell Tumour – Carcinoma | 1 | 0.7% |
| Endometrioid Tumour – Carcinoma | 1 | 0.7% |
Serous cystadenoma. a. cut section shows unilocular cyst with papillary excrescences. b. Histology showing a simple cyst lined by a single layer of ciliated columnar epithelium resting on a fibrocollagenous stroma (H & E, 40X).
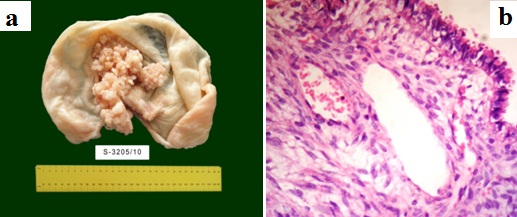
Mucinous cystadenoma a. Cut section showing a multilocular cyst with smooth, glistening inner surface. b. Histology showing a cyst lined by columnar cells with basal nucleus and apical mucin, resting on fibrocollagenous stroma (H & E, 40X).
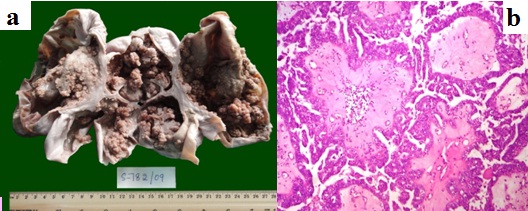
Papillary serous cystadenocarcinoma. a. Cut section shows multiloculated cyst with intra-cystic papillary excrescences and complex branching papillae. b. Histology shows papillary architecture with thick fibrovascular core, stratification and nuclear pleomorphism (H & E, 40X).
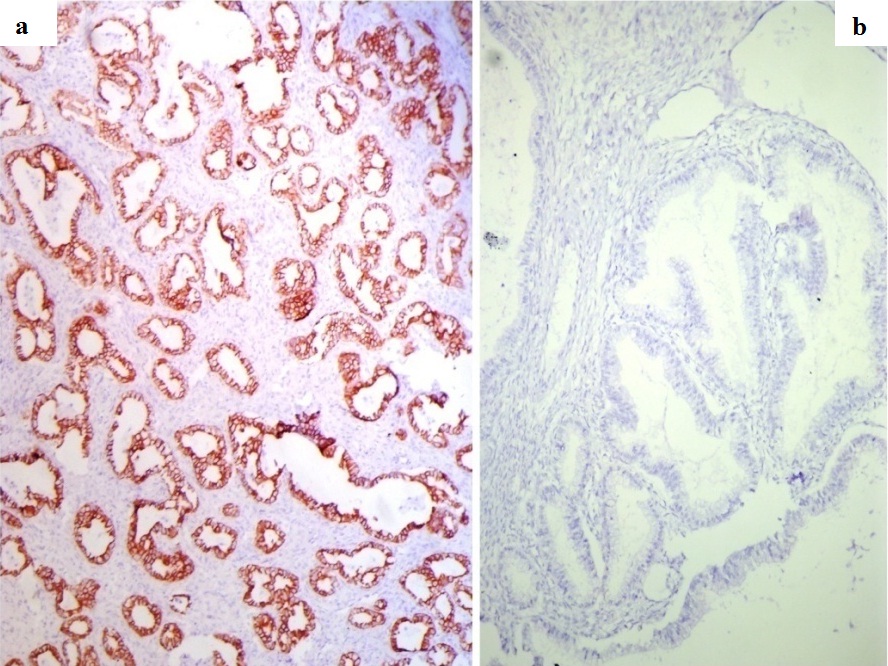
Mucinous carcinoma – IHC. a. Strong epithelial membrane positivity for CK7(IHC, 10X). b. CK 20 negative (IHC, 10X).
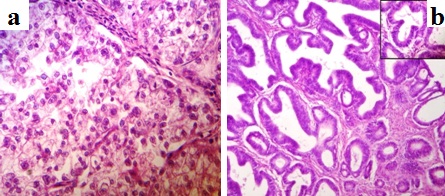
a) Clear Cell Carcinoma - Histology shows solid sheets and tubulopapillary pattern of cells with abundant clear cytoplasm separated by delicate fibrovascular septae (H & E, 40X). b) Endometrioid Carcinoma - Histology shows confluent glandular pattern with back to back appearance and minimal stroma in between (H & E, 10X). Inset – Higher power view of the same (H & E, 40X)
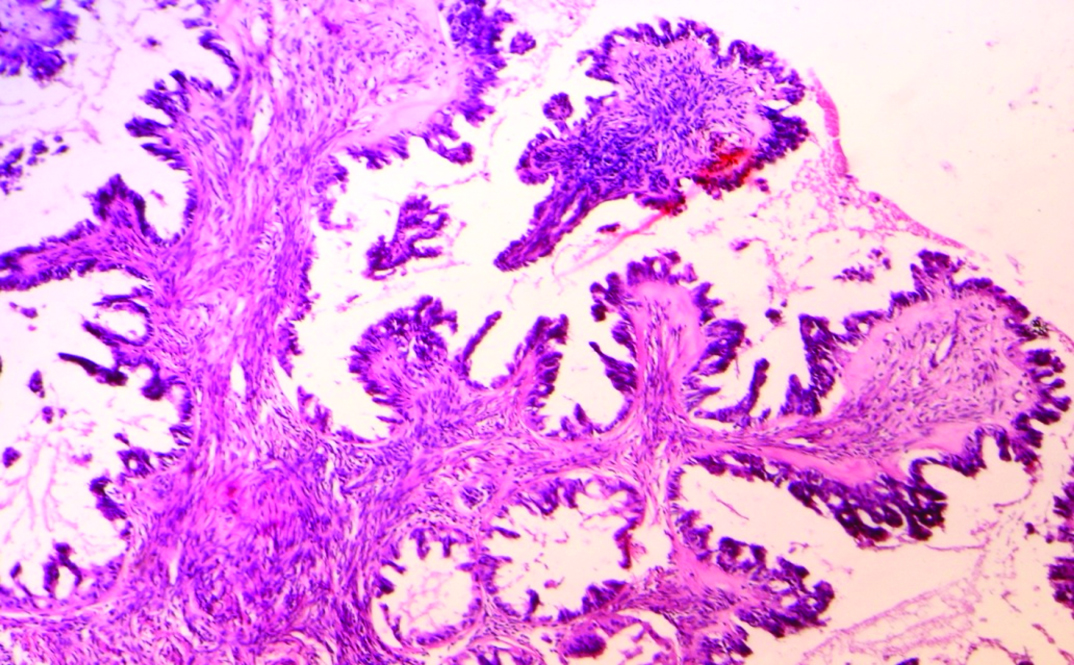
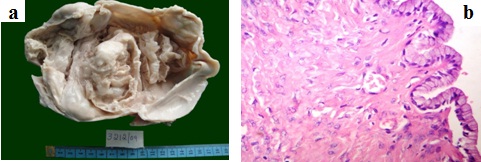
Borderline serous tumour. Histology showing hierarchial branching pattern with thick papillae. Lining epithelium showing budding, stratification and cytologic atypia (H & E, 10X).
Age distribution and clinical presentation of surface epithelial tumours.
| Age distribution (years) (%) | Clinical Presentation (%) |
|---|
| Mass in abdomen | 58.9 |
| Pain abdomen | 35.5 |
| <20 | 2.8 | Abnormal bleeding Per Vagina | 8.7 |
| 20-29 | 15.8 | Post menopausal Bleed | 4.8 |
| 30-39 | 18 | Ascitis | 14.9 |
| 40-49 | 35.3 | Fever | 0.7 |
| 50-59 | 15.1 | Gastrointestinal tract symptoms | 2.8 |
| 60-69 | 6.5 | Genitourinary symptoms | 1.4 |
| >70 | 6.5 | Infertility | 1.4 |
| Asymptomatic | 11.3 |
External surface, locularity and frequency of papillary projections.
| Type of tumor | External surface (%) | Locularity (%) | Papillary projections (%) |
|---|
| Smooth | Nodular | Unilocular | Multilocular |
|---|
| Serous | 60.2 | 28.9 | 68 | 32 | 58 |
| Mucinous | 54.3 | 43.5 | 15 | 85 | 4.3 |
A case of synchronous primary malignant tumors of ovary, fallopian tube and cervix was encountered in the study. One case showed serous cystadenofibroma on the right and serous cystadenoma on the left. Other case had papillary serous cystadenoma on the right and borderline serous on the left. A mixed epithelial tumour with serous cystadenofibroma along with a minor component of brenner tumour was also seen in this study.
Discussion
Surface epithelial tumours are derived from the ovarian surface epithelium which develops from the coelomic epithelium (modified mesothelium) which lines the ovary. This epithelium penetrates the underlying mesenchyme to form mullerian duct. The various histological types of ovarian surface epithelium tumours such as tubal type in serous neoplasms, endometrial type in endometrioid tumours and endocervical type in atleast some mucinous neoplasms are attributed to this embryonic proximity [5].
As women approach menopause, the ovarian surface epithelium often extends into the underlying stroma to form inclusion glands, which may become cystic. Surface epithelial tumours can arise directly from the surface epithelium and can grow outwardly, but more often they originate from inclusion glands, thus, accounting for the cystic (endophytic) nature of most of these tumours. These tumours become solid when they contain a large stromal component or when malignant cells within them proliferate [7,8].
Most of these tumours are composed of more than one cell type. They are usually classified based on predominant cell type. When the tumours are composed of two or more of the five major cell types (serous, mucinous, endometrioid, clear cell and transitional) and the 2nd or 3rd predominant cell type together account for > 10% of tumour epithelium, they are termed as mixed epithelial tumours [5].
Differentiation and extent of proliferation of the epithelium form the basis of classification of surface epithelial tumours. The most important group of neoplasms, which arises from epithelial tumours are classified according to following parameters [9]: 1) Cell type: serous/ mucinous/ endometrioid/ clear; 2) Pattern of growth: cystic /solid; 3) Amount of fibrous stroma; 4) Atypia and invasiveness: benign/ borderline/ malignant.
Epidemiological studies of ovarian cancer rely on accurate tumour classification [7]. In 1973, WHO gave a classification based on gross features, which is clearly devoid of any validity. In late 1980, International Society of gynaecologic pathologists proposed a classification which was based on histogenesis, which is now adopted as WHO classification [6,7].
Advances in molecular biology correlated with morphologic studies and have led to the proposal of a new model of carcinogenesis with important clinical implications. Based on clinicopathologic features and characteristic molecular genetic changes, surface epithelial tumours were typed into two broad categories – Type I and Type II. This does not refer to the histopathologic diagnostic terms [8,10,11]. As genetic analysis was not carried out in our study, due to financial constraints, this typing was not incorporated in the present study. Type I tumours are low grade, relatively indolent neoplasms arising from well characterized precursor lesions (borderline tumours and endometriosis). This group includes low grade serous tumours (invasive micropapillary serous carcinoma), low grade endometrioid tumours, tentative clear cell carcinomas and mucinous carcinomas. These tumours harbor somatic mutations of genes encoding protein kinases including KRAS, BRAF, PIK3CA and ERBB2 and other signaling molecules namely PTEN and CTNNB1 (β catenin). Mucinous and borderline serous tumours seem to arise from cystadenomas while clear cell tumours and borderline endometrioid tumours arise from endometriosis (endometriotic cysts and endometriomas) [11].
Type II tumours are aggressive, high grade neoplasms which arise denovo. They include high grade serous carcinoma which are said to arise from intra-epithelial carcinomas, majority of which have been detected in tubal fimbriae. These tumours have TP53 mutations, which are interestingly detected in tubal intra-epithelial carcinoma designated recently as “p53 signature” [10,11]. It is important to recognize type II tumours, as they account for vast majority of ovarian cancer deaths.
Progression of ovarian cancers is poorly understood. Presumption that carcinoma arises in the ovary and is confined to the ovary for sometime before disseminating into pelvis, abdominal cavity and distant sites forms the basis of International Federation of Gynaecology and Obstetrics (FIGO) staging system[11]. Study of precursor of ovarian cancers is difficult as ovaries are not readily accessible for screening, and identification of putative precursor lesion is based on the microscopic examination of resected ovary. Hence, natural history of lesion cannot be observed [5,11].
Surface epithelial tumours account for approximately two thirds of all ovarian neoplasms and their malignant forms represent 90% of all ovarian cancers [5]. In our study, surface epithelial tumours accounted for 66.2% of ovarian tumours, which is consistent with the studies done by Zaman et al., [12]. Age is described as an independent prognostic factor in ovarian tumours [12]. Ovarian cancer rates increase exponentially with age [11]. Approximately 1 in 8 tumours in patients aged <45years is malignant, which by contrast increases to one in 3 in older women. Borderline tumours are seen in women in their 30’s. The mean age of diagnosis in our study was 42.4 years. The age varied from 14-76 years [Table/Fig-8]. The age distribution was comparable with studies done by Zaman et al., Pilli et al., Jha et al., Kayastha et al., and Mankar et al., [12,13–16].
In our study, mass abdomen seen in 58.9% cases was the most common presenting symptom followed by pain abdomen (35.5%) [Table/Fig-8]. Malignant tumours most commonly presented with ascitis and mass abdomen. These findings were comparable with the studies done by Maheshwari et al., [17]. Pain abdomen was the most common finding in the study done by Mankar et al., [16]. Menstrual irregularities were more common in the study done by Kanthikar et al., [18].
The laterality of ovarian cancers is a clue to their nature [19]. Bilaterality is a common feature of metastatic tumours and an important diagnostic clue. But, one has to be cautious while diagnosing them, as typical serous or undifferentiated carcinomas can also be bilateral [5]. In our study, majority of the cases (90.6%) were unilateral. Kanthikar et al., also reported higher incidence of unilateral tumours in their study [18].
Right sided tumours were more common compared to the left sided tumours in our study and this finding was consistent with the studies done by Pilli et al., Ramachandra et al., and Saxena et al., [13,20,21]. Study done by Madan et al., reported a higher incidence of left sided tumours [22].
Benign serous tumours are usually small and the mucinous tumours present as huge masses [9]. The smallest tumour in our study was a serous cystadenoma measuring (3x2x1)cm and the largest tumour was mucinous cystadenoma measuring (43x30x5)cm. Careful examination of the external surface, sectioned surface along with appropriate sampling is essential for rendering a correct diagnosis. Benign serous tumours have a smooth external surface since majority of them are unilocular, while mucinous tumours show a nodular external surface owing to their multilocular nature [7,9]. Malignant tumours exhibit breach of capsule and variegated appearance with predominant solid areas with hemorrhage and necrosis, while benign tumours do not [4,9]. The same was observed in our study also. Papillary projections on cut section can be seen in benign, borderline or malignant tumours [4,7,9]. In the present study, they were seen more frequently with serous tumours (58%) than with mucinous tumours (4.3%).
Like all other tumours even surface epithelial tumours of ovary are differentiated based on atypia and invasiveness as benign and malignant tumours. In addition, they also exhibit an intermediate borderline category referred to as “tumours of low malignant potential” [6]. These are low grade cancers with limited invasive potential and hence, have better prognosis than fully malignant ovarian carcinomas. In our study, 82.3% were benign tumours, 12.1% malignant tumours. 5.7% tumours belonged to the borderline category. In comparison with the studies done by Zaman et al., Pilli et al., Jha et al., Mankar et al., Maheshwari et al., Forae GD et al., and Dhawar et al., [12,13,14,16,23,24], the incidence of malignant tumours in our study was low. This is probably because the study was undertaken in a general hospital where malignant tumours when diagnosed before surgery get referred to speciality oncology centers.
Histopathologic diagnosis of ovarian tumours is based on pattern- cell- type approach. Based on the cell types, we had 61.7% serous tumours, 34.8% mucinous tumours, one clear cell carcinoma, one endometrioid carcinoma and 3 benign transitional cell tumours. The frequency of occurrence of these tumours were comparable to the studies done by Kanthikar et al., Dhawar et al., Kar Tushar et al., and Swamy et al., [18,24–26].
Ancillary techniques like Immunohistochemistry which are sparsely used in diagnosis of ovarian tumours, finds its use here to distinguish primary ovarian mucinous carcinoma from metastatic colorectal adenocarcinomas. Ovarian mucinous carcinomas (intestinal type) show strong positivity when stained with Cytokeratin (CK)7 and exhibit variable positivity for CK20. Colorectal carcinomas stain strongly with CK20 but do not stain with CK7 [27,28]. Two cases in our study were diagnosed based on CK7 positivity and CK20 negativity.
We had a case of triple synchronous primary malignancies of female genital tract comprising primary cystadenocarcinomas of fallopian tube and ovary with adenosquamous carcinoma of the cervix. Synchronous multiple tumours of female genital tract are rare, comprising only 1.6% of genital neoplasms [29]. Cases of triple synchronous primaries are extremely rare with only 4 cases reported till date involving cervix, ovary and endometrium. This is the first reported case of synchronous malignancies of fallopian tube, ovary and cervix.
Limitation
1. Genetic analysis was not carried out due to financial constraints. 2. Study was undertaken in a single general hospital where malignant tumours, when diagnosed before surgery get referred to speciality oncology centers.
Conclusion
Advances in molecular biology and large volumes of literature on use of ancillary techniques for better understanding of surface epithelial tumours are on the rise, but still morphological study by histopathological techniques are still the backbone for diagnosis of these tumours.