To make the prepared root canal as bacteria free as possible, it is believed that mechanical enlargement of canals must be accompanied by copious irrigation and chemical disinfection by intra-canal medication [1]. The irrigating solution should provide antibacterial effects and also provide disinfection in areas of the canal that is inaccessible to mechanical instrumentation. But even after conventional canal instrumentation, certain microorganisms remain within the dentinal tubules, smear layer or apical dentinal plug.
One of the main cause of root canal therapy failure is the persistence of microorganisms that cause reinfection. E. faecalis, was found to be the most prevalent bacteria, and the predominance of this organism was found to be much higher in root filled teeth (67%) than primary endodontic infections (18%) [2].
Currently sodium hypochlorite is the choice of irrigant preferred by many clinicians. But it causes severe inflammation and cellular destruction in all tissues and when extrusion occurs through the apices of teeth, causes severe pain, swelling and necrosis [3].
Because of recognized limitations of all endodontic irrigants available in the market, there is a need for developing an irrigant with broad antibacterial spectrum, rapid disinfection time, limited toxicity and an environment friendly irrigant is always a desirable property.
This paper investigated the antimicrobial efficacy of Electro-chemically Activated (ECA) water against commonly available and commonly used root canal irrigant sodium hypochlorite.
1. Anolyte (with oxidation potential): The anolyte is a brownish, acidic water with a characteristic odour and taste. The anolytes are of two types: A-Acidic Anolyte (pH<5;ORP= +800...+1200Mv, the active components-HClO, Cl2, HCl, HO2; AN-Neutral Anolyte (pH=6.0, ORP=600...+900mV), the active components–HClO, O3, HO-, HO2. They have proven anti-bacterial, anti-viral, anti-fungal, anti-allergic and anti-inflammatory action with least toxicity to the human tissue cells.
2. Catholyte (with reduction potential): Catholyte exists in a metastable or disequilibrious state after production and can be of two types: C–Alkaline Catholyte (pH > 9,0; ORP = -700– - 820mV), the active components–2 molecules of O2 and HO2-, NaOH, HO2*, OH-, OH*; CN–Neutral Catholyte (pH = 9,0; ORP = - 300 – -500mV), the active components – O2, HO2-, HO2*, H2O2, H+,OH-. Catholyte has weaker antimicrobial property compared to anolyte [7].
The aim of the study was to compare and evaluate the antimicrobial effectiveness of ECA, 1% hypochlorite, and 3% hypochlorite when tested against the standard strain of E. faecalis (ATCC 29212).
Materials and Methods
This in-vitro study was performed in the Department of Conservative Dentistry and Endodontics, Kalinga Institute of Dental Sciences, and Department of Microbiology, Kalinga Institute of Medical Sciences, Bhubaneswar, Odisha, India. The duration of the study was approximately 6 months.
Forty eight freshly extracted, human permanent maxillary central incisors of patients between the age group 40 to 60 years undergoing extractions due to periodontal reasons were selected for the study from the Department of Oral and Maxillofacial Surgery.
Single rooted, fully matured, non-carious maxillary central incisors having intact radicular portion without any resorptive defects were included in the study and teeth with surface defects, fracture lines in the roots or any resorptive defects were excluded.
All the teeth were decoronated at Cemento-Enamel Junction (CEJ), to obtain approximately 15mm length. The apices of the teeth were sealed with Glass Ionomer Cement (GIC). The remaining root surface was coated with a double layer of nail varnish. The roots were cleaned and shaped to ISO size of 40-K file, and copious irrigation with 3% sodium hypochlorite and 17% EDTA was done for 5 minutes, there by filling them with a creamy mixture of calcium hydroxide powder. A sterile cotton pellet was placed into canal orifice and the access cavity was sealed with a re-inforced zinc-oxide eugenol material (IRM, Caulk, Dentsply). After this, the samples were stored in distilled water at 4oC for a week until further use as at this temperature no bacterial growth could take place. The 48 samples were divided into four groups of 12 each. They were mounted in sterilized micro-tip boxes made up of polypropylene, and then they were sterilized in an autoclave.
Only 1ml of sterile Ringer’s solution was taken in a tuberculin syringe and injected into the root canal of each tooth sample and aspirated. This was repeated for three times, the third and final aspirated Ringer’s solution was placed onto a blood agar plate and was spread. The plates were incubated aerobically at 370C for two days. All cultures were negative after two days. This was done to confirm negativity.
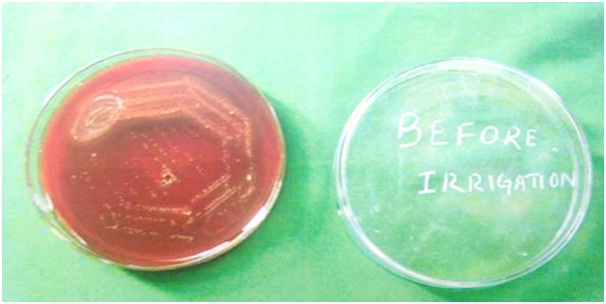
Then a calibrated suspension of E.faecalis was prepared and inoculated into 50ml of Brain Heart Infusion (BHI) broth to form a suspension. Bacterial suspension was inoculated into each root canal, using the tuberculin syringe. Then the samples were incubated aerobically at 370C, in a Biological Oxygen Demand (BOD) incubator for 48 hours. After two days the micro-tip boxes were removed, the suspension inside the canal was aspirated and 50 micro litres was spread onto marked blood agar plates [Table/Fig-1]. The plates were incubated at 370C for 48 hours and the presence of organism inside root canals of each tooth was confirmed. The CFU counts varied from 800 to 1050μL. This was done to confirm positivity.
Blood agar image before irrigation.
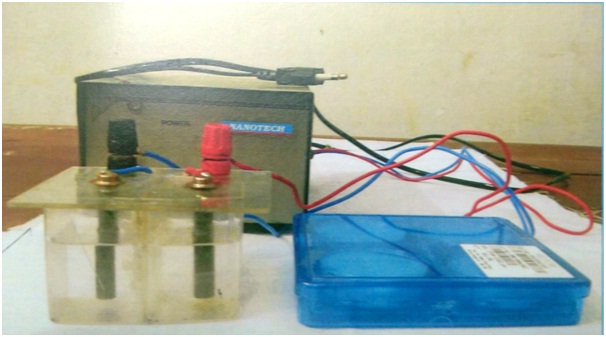
Preparation of ECA: The solution was prepared by using a custom-made apparatus which was made of two chambers capable of storing 50ml of the solution. The two chambers were separated from each other by a polymer membrane (M.F. Millipore) of pore size 0.45 micrometer. Connected to the top of the compartments were two graphite electrodes, 6cm long and 1cm in diameter, which were linked to a power supply that was capable of delivering a maximum of 12.5Volt and 3.5Amp. Only 40ml of tap water was poured into each compartment of the apparatus which led to electrolysis of water, and the solution obtained in anodic compartment was collected [Table/Fig-2]. The pH of solution was found to be 6.81 (Anolyte/Neutral), using a digital pH meter.
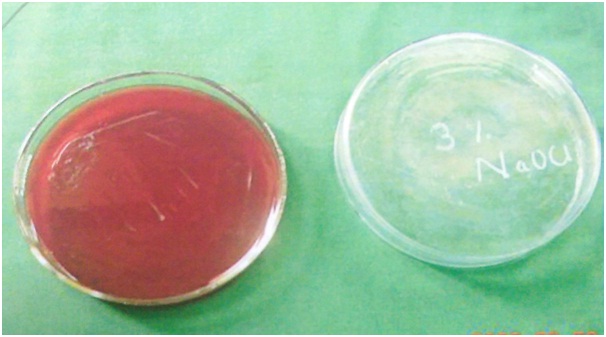
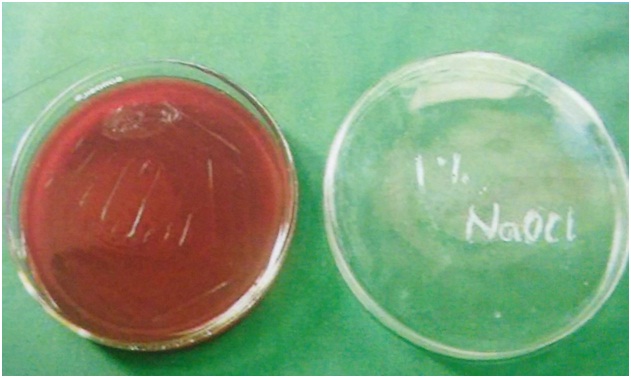
The root canals of all the teeth samples were irrigated with four different testing irrigating solutions (2ml) for 5 minutes. Directly following irrigation, BHI solution was injected into each canal and aspirated immediately. The third aspirated BHI solution (50μL) was spread onto a blood agar plate and incubated aerobically at 37OC for 48 hours [Table/Fig-3,4,5 and 6]. After inoculation, the CFU counts for all the teeth were done using an optical microscope. The counts obtained were on an average of 19 per 50 microlitres in all the teeth samples.
Blood agar image of 3% sodium hypochlorite.
Blood agar image of 1% sodium hypochlorite.
Blood agar image of distilled water.
Four test solutions were grouped according to
Group A : ECA (anolyte solution)
Group B : 1% sodium hypochlorite (prepared by adding distilled water and 3% NaOCl in 2:1 ratio)
Group C : 3% sodium hypochlorite (commercially available)
Group D : Distilled water
Spectrophotometric Analysis: Following the procedure, 50μL of BHI solution was aspirated from each root canal of all the groups, incubated into 5ml of BHI broth and optical density was measured (concentration of the bacteria), using Jenway, 6505, Spectrophotometer. The measurement was done for a particular wavelength (253nm) absorbed by the E. faecalis organism. Spectrophotometric analysis and the optical density values correlated well with the CFU counts obtained.
Statistical Analysis
The results were submitted to one way ANOVA, performed as parametric test to compare different variables, taken before or after irrigation, as well as to Dunkan’s Multiple Range Test as post hoc analysis to elucidate the difference between each group for all statistical analysis evaluations, a two tailed probability of value, p< 0.05 was considered significant. The data were expressed as means and standard deviation.
Results
When the tooth samples were subjected to CFU counts after inoculation with E. faecalis, the values were in the range of 800 to 1050 per 50 micro litres in all the teeth sample. It is the base line data of the entire 48 teeth sample irrespective of groups. They were further divided into four groups just before irrigation with test irrigation materials.
Colony forming unit values among individual groups after irrigations were compared, and the difference was found to be not statistically significant between Group A (ECA), Group B (1% sodium hypochlorite), and Group C (3% sodium hypochlorite). Though 3% sodium hypochlorite shows slightly better result than ECA, or 1% sodium hypochlorite, the difference was not statistically significant [Table/Fig-7]. The optical density values from the spectrophotometer analysis were compared before and after irrigation and were found to be statistically significant [Table/Fig-8].
Analysis of variance (one way ANOVA) of CFU count (Count X 10-4) of different treatment before and after irrigation.
| Group | Mean (μL) | ±SD | F value | p-value |
|---|
| Before Irrigation | 977.192b | 77.520 | 1566.495 | <0.001 |
| After Irrigation: Distilled H2O | 963.192b | 128.773 |
| After Irrigation: ECA | 19.808a | 6.499 |
| After Irrigation: 1% Hypo | 17.885a | 3.536 |
| After Irrigation: 3% Hypo | 10.808a | 4.040 |
a,b – Means with same superscript do not differ each other (Duncan’s Multiple Range Test)
Analysis of variance (one way ANOVA) optical density (Spectro-photometer) of different treatment before and after irrigation.
| Group | Mean (nm) | ±SD | F value | p-value |
|---|
| Before Irrigation | 0.452c | 0.002 | 47530.367 | <0.001 |
| After Irrigation: Distilled H2O | 0.462c | 0.002 |
| After Irrigation: ECA | 0.102b | 0.003 |
| After Irrigation: 1% Hypo | 0.081a | 0.002 |
| After Irrigation: 3% Hypo | 0.066a | 0.002 |
a, b,c – Means with same superscript do not differ each other (Duncan’s Multiple Range Test)
The optical density values were also compared between individual groups, and the results showed that, the difference between Group B and C were not significant. But, there was a statistically significant difference between the optical density values among Group B and C, and Group A.
Discussion
How ECA Water Kills Microorganisms: The ORP of ECAa (ano-lyte) is +800 mV to +1,200 mV, which creates an environment outside the working range of important microbial metabolic processes [7]. When immersed in these solutions, the microorganisms are exposed to powerful oxidants causing the rupturing of biochemical bonds and hence leading to loss of cell function. Moreover, the high ORP environment creates an unbalanced osmolarity between the ion concentrations in the solution and that within unicellular organisms, further damaging membrane structures [8]. This leads to increased membrane porosity, enabling oxidizing moieties present in ECAa (anolyte) to penetrate into the cell cytoplasm, ultimately leading to the inactivation of intracellular protein, lipids and nucleic acid, rendering the cell non-functional [9–12].
Antimicrobial Components of ECA Water: According to many studies, it was concluded that HOCl is the primary active agent present in acidic ECAa (anolyte) [13–15] which disrupts many microbial structure [16,17]. In addition, OH- hydroxyl radicals, which is the strongest oxidizing agent also have shown antimicrobial activity [16,17].
Most commonly used root canal irrigant in any clinical set up is sodium hypochlorite and to test the antimicrobial efficacy of any new root canal irrigant, sodium hypochlorite always acts as standard of comparison. This study also uses the same protocol of comparison.
Since 2001, as well as in earlier studies many researchers had concluded that antimicrobial property of ECA water is questionable, and they concluded that ECA water has only limited activity as compared to other routine treatments e.g., EDTA or sodium hypochlorite [18–20]. Many researchers have again reinvented the exact mechanism of action of ECA water as an antimicrobial root canal irrigant yet retaining its mildness and being less toxic. The present study was chosen only to re-evaluate the antimicrobial efficacy of ECA water.
A comparative study by Gulabivala et al., in 2004, found that bacterial culture study for CFU showed the hydroxyl ions in ECA water reduce the biofilm formed by E. faecalis in infected tooth models [18].
More recently, there has been increased interest in pH-neutralized ECAa (anolyte with a pH 6-8) as an antimicrobial solution (e.g., Sterilox™ and Microcyn™), and have shown broad-spectrum bactericidal activity [21,22]. Neutralized ECAa is thought to be more beneficial because of its biocompatibility and longer shelf life. The present study utilizes neutral anolyte solutions with pH 6.81. In another study, which compared antimicrobial activities of chlorine matched (100ppm) aerosolized sodium hypochlorite, chlorine dioxide and electrochemically activated solutions, concluded that ECA was a better disinfectant than sodium hypochlorite [23].
RMS Thorn et al., 2011 in a study had briefed the advantages and disadvantages of ECA which are as follows [24]:
| Advantages | Disadvantages |
| Broad spectrum anti microbial activity | Initial expenditure on the generator |
| Rapid disinfection time | Generator servicing and maintenance |
| Inexpensive, on site or in situ generation, | Limited shelf life |
| Easily accessible raw materials | Inactivated by organic loading |
| Limited toxicity and environmental friendly | ECAs can be corrosive |
Under the conditions of the present study, the irrigating solutions failed to destroy all the bacteria within the root canals. But all the three experimental groups considerably reduce the bacterial count (CFU). Comparable results were obtained with ECAs, 1% sodium hypochlorite and 3% sodium hypochlorite groups. Though 3% sodium hypochlorite showed slightly better results, there was no statistical difference between the three groups.
Spectrophotometric analysis of all the samples had also been done to obtain a qualitative value of bacterial concentration. The optical density values before and after irrigations were compared for all the groups. The results showed a statistically significant difference in all three experimental groups, compared before and after irrigation. The results were also compared between individual Groups, and the results showed that the difference between group B (1% sodium hypochlorite) and C (3% sodium hypochlorite) were not significant. But there was a statistically significant difference between the optical density values among Groups A and Group B and also between Group A and Group C. Hence, the results obtained in ECA showed a significant difference from both 1% and 3% group. Though the numerical values appeared quite similar, but the difference between the ECA and sodium hypochlorite in spectrophotometric analysis was significant.
Under clean conditions freshly prepared ECA water was found to be highly active against all microorganisms giving 99.999% or greater reductions in less than two minutes, thus many investigators concluded ECA water as potent anti-microbial agent and its clinical application were reported to be effective which is in accordance with our study [25,26]. In another comparative study by Helme AJ et al., in 2010, the bactericidal activity of ECA water was found four times more effective than commercially available 2.5% sodium hypochlorite solutions [27].
According to another study, irrigation with 3% sodium hypochlorite and ECA water enhanced the opening of dentinal tubules, irrigation with sodium hypochlorite resulted in with more open tubules in the coronal and middle third of canal whereas with ECA water it resulted in more numerous open dentin tubules in apical as well as coronal third [28].
Limitation
One must bear in mind that bacterial studies in-vitro/ex-vivo are quite different when compared to dynamically complex biological system that occurs in root canal system (in-vivo). Thus, direct extrapolation to clinical conditions must be exercised with caution because of obvious limitations. The discrepancies in the literature are likely due to the method of delivery, as this has been suggested to be the critical treatment factor.
Although the use of ECA water as root canal irrigant has shown early promise, it is still in its infancy stage. It needs further studies and clinical trials before using it in true clinical situations.
Conclusion
ECA water being non-toxic, more biocompatible, inexpensive, easily available and a potent antibacterial agent, it can be very well used as a root canal irrigating solutions. The antibacterial efficacy of ECA water was found to be comparable with sodium hypochlorite solution against E. faecalis.
a,b – Means with same superscript do not differ each other (Duncan’s Multiple Range Test)
a, b,c – Means with same superscript do not differ each other (Duncan’s Multiple Range Test)