Introduction
Autism is a neuro-developmental disorder which is manifested as impairment of social interaction, communication and a repetitive behaviour. Autism can obscure dental treatment for the affected patients; furthermore, children with autism commonly have destructive oral habits.
Aim
The aims of this study were to evaluate the Modified Gingival Index (MGI), Plaque Index (PI), salivary pH and buffering capacity of the saliva among autistic children compared to normal children in Riyadh City that may provide baseline data to enable comparison and future planning of dental services for autistic children.
Materials and Methods
A total of 50 children diagnosed with autism (mean age 8.5 years) were selected from Azzam Autism School, Riyadh City. The control group consisted of 50 non-autistic school children (mean age 8.7 years), gender matched, selected from Outpatient Clinic, Riyadh Colleges of Dentistry and Pharmacy. MGI, PI, salivary pH and salivary buffer capacity tests were done for all participants. The buffering capacity of the stimulated saliva was grouped under ‘very low’, ‘low’ and ‘normal’. Pearson’s Chi square and one way ANOVA were used to find statistical significance if any among the autistic and the normal control group.
Results
The results of the study showed that the mean ± standard deviation of MGI, PI and pH of unstimulated resting saliva for autistic group were 1.82 ± 0.65, 1.92 ± 0.35 and 6.8 ± 0.5 respectively. Normal control group had values 1.35 ± 0.85, 1.44 ± 0.43 and 7 ± 0.4 respectively. A statistically significant difference between both groups for all parameters was found. Salivary buffering capacity was found to be normal for the majority among both groups. However, 60% children among the autistic group presented with normal buffering capacity of the stimulated saliva as compared to 70% among the normal control group. However, this difference was not statistically significant (p = 0.544).
Conclusion
Children with autism appear to have higher gingival inflammation, poor oral hygiene and a slightly lower salivary pH as compared to healthy control group. Special oral health programmes regarding treatment and maintenance of good oral health should be taken in consideration for autistic children.
Autism, Buffering capacity, Gingival index, Plaque, Saliva
Introduction
Autism is defined as a neuro-development disorder that is characterized by impairment of social interaction, communication and a repetitive behaviour [1]. Autism is a highly variable brain development disorder with unknown aetiology that first appears during infancy or childhood [2] .
The prevalence of autism is estimated to be 1-2 per 1,000 involving about four times males as compared to females [3]. The predominance of autism in males has been reported to be as high as 82.8 % [4]. Prevalence estimates seem to have increased over time and these findings most likely represent broadening of the diagnostic concepts, service availability, and awareness of Autistic Spectrum Disorders (ASD) in both the normal and the professional public. Also, unidentified environmental risk factors cannot be ruled out as other contributing factors [5].
Clinically, autism is defined by a “triad” of deficits consisting of impaired social interaction, impaired communication, restricted interests, and repetitive behaviour. Also in some patients speech may not develop fully or may be meaningless in a normally paced conversations [6]. Although, it has been observed that people with ASD are more likely to be caries-free than their unaffected counterparts; yet, more patients with ASD are uncooperative and require general anesthesia for dental treatment [7]. Moreover, children with autism also commonly have destructive and detrimental oral habits such as bruxism, tongue thrusting, picking at the gingiva and lip biting [8]. It has also been proposed that autistic children have preference for sweet food which makes them more vulnerable for dental caries [9].
There are few studies describing gingival health and salivary parameter of children with autism. Some of them reported statistically significant differences in the prevalence of caries, gingivitis and degree of oral hygiene in comparison with non-autistic individuals [8,10–12]. While as, other studies reported no significant differences in autistic patients [13,14]. Autistic group like other special needs groups presents a high unmet dental need [15]. Nevertheless, the dental professional should be flexible to modify the treatment approach according to the individual patient needs [16].
Therefore, the aim of the present study was to evaluate the gingival health by means of gingival index and plaque index among autistic and non-autistic children. The present study also looked at salivary parameters like pH of unstimulated saliva as well as the buffering capacity of the stimulated saliva among the groups. These parameters can be of prime importance in making an individual susceptible or resistant to dental diseases like caries.
Materials and Methods
Study Design and Study Population: This study was a case control study. Out of 200 students enrolled at Azzam Autism School, Almorsalat Square, Riyadh City; 145 names were tabulated from the registration numbers of the school register after the application of the inclusion and exclusion criteria. Eventually a total of 50 patients with Autism (mean age 8.5 years) were selected by random sampling using random number tables. The control group consisted of 50 non-autistic (mean age 8.7 years), gender matched controls, selected from the outpatient clinic, Riyadh Colleges of Dentistry and Pharmacy. The following parameters were evaluated for every patient:
Modified Gingival Index described by Lobene et al., 1986 [17,18]
Plaque Index described by Silness and Löe 1964 [19]
Salivary pH of the resting saliva using pH-Fix test strips [Macherey-Nagel CE 3.1-8.3]
Buffering capacity of the stimulated saliva using GC’s Saliva-Check Buffer kits
The study was conducted between August 2015 and December 2015. There are specialized centres for autistic people in three main cities of Saudi Arabia [20]. The study describes one specific type of special school for autistic children and adolescents, Azzam Autism Centre and Special School for Autistic Children, Riyadh. The participants in the case group were 4 to15 years old. The subjects were diagnosed mostly as autistic with or without learning disability. Potentially uncooperative patients were excluded from the study and eventually a total of 50 subjects were enrolled in this study. The inclusion criterion for the case group was a diagnosis of autism. The exclusion criteria set was a dental prophylaxis in the last 6 months, patients with systemic disorders that affect the periodontal disease and diabetes. Controls were equally matched in terms of age and gender. All controls included in the study were medically fit and were not undergoing antibiotic or anti-inflammatory therapy or had not undergone dental prophylaxis in the past 6 months.
Ethical Approval: The proposal of the project was presented to the Chair of the Research Ethics Committee, at Riyadh Colleges of Dentistry and Pharmacy, Riyadh, Saudi Arabia. The parents of the participants were given participant information letters in the local language (Arabic), distributed from the centre. After fulfilling all these requirements and in accordance to International Guidelines [21] a formal ethical approval was granted vide RCDP-NFR 5775/2015. An official permission was also obtained, from the principal of the special needs school.
Training and Calibration: Before the commencement of the study, the examiners were standardized and calibrated in the Department of Paediatric Dentistry, Riyadh Colleges of Dentistry and Pharmacy, Riyadh to ensure uniformity in the collection of data. Two examiners underwent training for clinical examination using the equipment while two others underwent training exercise to record data for one week. Overall reliability of the examiners was assessed after two weeks. Furthermore, calibration was done at the third week to seek the intra-examiner and inter-examiner variability. Overall Kappa score of 0.96 was achieved for intra-examiner variability and 0.90 for inter-examiner variability.
Methodology: All subjects were examined by two examiners using dental mirror, explorer and Marques periodontal probe. Each examiner was assisted by a person who recorded the scores on Ramfjord teeth. Each child was accompanied by his/her parents. MGI as described by Lobene et al., [17] does not require dental equipment for recording. The scores can be recorded visually. The codes range between 0-4. Each tooth was scored on the basis of inflammation present or absent in the accompanying gingiva. The overall score was divided by the total number of teeth examined. The overall mean of the group was eventually calculated. Plaque Index (PI) scores range between 0-3 from absence to abundance of plaque. Marques periodontal probe was used to assess the plaque index of the groups. The score for the four areas of the tooth was summed up and divided by four to yield a total tooth score (vestibular, medial, oral, and distal) [19]. By adding the tooth scores together and dividing by the number of teeth examined, the patient’s PI was obtained. Similar to the MGI, the overall mean of PI for both groups was calculated. The pH of resting saliva was calculated using suction apparatus. The pH-fix strips 3.1-8.3 were used for estimation of pH of resting saliva for both groups. GC’s salivary buffer strips were used to estimate the buffering capacity of the saliva. Stimulated saliva was collected from the subjects by asking them to chew a paraffin wax tablet prior to the collection. The salivary buffering capacity was categorized into ‘very low’, ‘low’ and ‘normal’ according to the colour change from red, yellow and green respectively on the strip.
Data Recording: Each examiner was accompanied by a person who recorded data for each subject and recorded the colour change in the pH-fix strips as well as GCs salivary buffering strips. All universal and standard precautions of infection control were considered and observed strictly for all subjects.
Statistical Analysis
The recorded data were compiled and entered in a computer using Statistical Package for Social Sciences (SPSS) version 20.0 software (Chicago, IL, USA). One way ANOVA and Chi-square tests were used for comparisons. A p-value of less than 0.05 was considered as statistically significant. The data was analysed comparing both groups. The descriptive statistics included the mean, range and standard deviation for both groups.
Results
The study evaluated the MGI, PI, pH of the resting saliva and salivary buffering capacity of the stimulated saliva among 50 autistic and 50 normal children. The age range in both groups was between 4-15 years. The average age among the autistic group was 8.5 years while as 8.7 years among the normal children group. Only 13 among the autistic group were girls. Same number of gender was selected from the normal control group. Since there was uneven distribution of gender, data was not analysed according to the gender. [Table/Fig-1] describes the minimum, maximum and mean scores along with the standard deviation for MGI, PI and pH for resting saliva. The mean of the MGI score in the autistic group (1.82) was higher than that of the normal children group (1.35). The mean MGI score of 1.82 in the autistic group was even higher than the mean (1.59) of the overall combined groups. However, the maximum MGI in autistic group (3.75) was slightly lesser than the maximum MGI score in the normal children group (3.87). Similarly, the PI mean score of the autistic group (1.92) was higher than that of the normal children group, 1.44, as well as the mean PI score of the overall children group (1.68). The salivary pH of the resting saliva in the autistic group was in the range of 5.4 – 7.8 with a mean pH of 6.8. The mean pH of the resting saliva in the normal children group was 7 with a range of 5.9- 7.9.
Shows the average (mean) scores of Modified Gingival Index (MGI), Plaque Index (PI) and the salivary pH (resting saliva).
| Test and Control Groups | Modified Gingival Index (MGI) | Plaque Index (PI) | Salivary pH |
|---|
| Autistic | N | 50 | 50 | 50 |
| Minimum | 0.87 | 1.25 | 5.4 |
| Maximum | 3.75 | 2.74 | 7.8 |
| Mean | 1.8254 | 1.9250 | 6.854 |
| Std. Deviation | 0.65343 | 0.35782 | 0.5545 |
| Normal | N | 50 | 50 | 50 |
| Minimum | 0.22 | 0.69 | 5.9 |
| Maximum | 3.87 | 2.73 | 7.9 |
| Mean | 1.3582 | 1.4426 | 7.088 |
| Std. Deviation | 0.85763 | 0.43056 | 0.4364 |
| Total | N | 100 | 100 | 100 |
| Minimum | 0.22 | 0.69 | 5.4 |
| Maximum | 3.87 | 2.74 | 7.9 |
| Mean | 1.5918 | 1.6838 | 6.971 |
| Std. Deviation | 0.79404 | 0.46249 | 0.5102 |
[Table/Fig-2] shows one way ANOVA of mean MGI, mean PI and the pH of the resting or unstimulated saliva in the autistic and the normal children group. In all the variables there was a statistically significant difference, p<0.05, between the autistic and the normal children group. However, the mean pH of the autistic group 6.8 was only slightly less than the overall pH of 6.9 and pH of 7 in the normal children group. [Table/Fig-3] compares graphically the mean MGI, mean PI and pH of the resting saliva among the autistic and the normal children groups.
Shows one way ANOVA of MGI, PI and resting salivary pH with respect to the autistic and normal groups.
| One Way ANOVA |
|---|
| Sum of Squares | df | Mean Square | F | Sig. |
|---|
| Modified Gingival Index | Between Groups | 5.457 | 1 | 5.457 | 9.388 | 0.003* |
| Within Groups | 56.963 | 98 | 0.581 | | |
| Total | 62.419 | 99 | | | |
| Plaque Index | Between Groups | 5.818 | 1 | 5.818 | 37.124 | 0.000 * |
| Within Groups | 15.358 | 98 | 0.157 | | |
| Total | 21.175 | 99 | | | |
| Salivary pH | Between Groups | 1.369 | 1 | 1.369 | 5.499 | 0.021* |
| Within Groups | 24.397 | 98 | 0.249 | | |
| Total | 25.766 | 99 | | | |
* Significant at p < 0.05
Shows graphical comparison of mean MGI, mean PI and mean salivary pH in autistic and normal children groups.
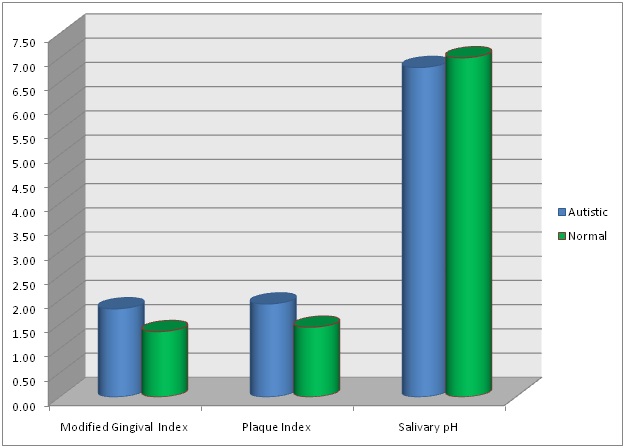
[Table/Fig-4] describes the salivary buffering capacity among the autistic and the normal children groups. The buffering capacity of the saliva was categorised as ‘very low’, ‘low’ and ‘normal’. There was no statistically significant difference of the salivary buffering capacity among the two groups (sig 0.54), p<0.05.
Shows the salivary buffering capacity of the stimulated saliva among the autistic and the normal children groups.
| Test and Control Groups | Salivary Buffer Capacity | Total | Sig. |
|---|
| Very Low | Low | Normal |
|---|
| Autistic | Count | 5 | 15 | 30 | 50 | |
| % within the group | 10.0% | 30.0% | 60.0% | 100.0% | 0.544 $ |
| Normal | Count | 3 | 12 | 35 | 50 | |
| % within group | 6.0% | 24.0% | 70.0% | 100.0% | |
| Total | Count | 8 | 27 | 65 | 100 | |
| % within Test and Control groups | 8.0% | 27.0% | 65.0% | 100.0% | Pearson’s Chi Square |
$ Not significant p > 0.05
Discussion
Behaviour of children with autism makes examination of oral hygiene and dental treatment a problem [10]. It has been shown that children with ASD experience more difficulties as compared to other normally developing children, they experience significant barriers to care in both the home and dental office [11]. The studied group showed more males than females, almost a ratio of 4:1, which might reflect the higher prevalence of autism in males as it was reported in other studies [2,3,22]. In the present study, children with autism exhibited a higher gingival and plaque indices and similar salivary buffer capacity compared to healthy control group. Similar results were found by Bassoukou et al., in 2009 [23] while as Rai et al., in 2012 found out that oral hygiene was poor in children with autism whereas the Salivary total antioxidant capacity was significantly reduced in autistic children [24].
Higher scores of MGI particularly in the autistic group may be related to poor oral hygiene. It has been shown that autistic children have significantly poor oral hygiene and higher incidence of malocclusion and dental caries when compared to other oral conditions [25]. These differences may be attributed to irregular use of brushing habits, owing to the behavioural problems associated with autism, which result in inadequate tooth brushing. It has been reported that people with ASD may reflect poor dental awareness, a lack of dental education and deficiency in receiving oral hygiene instructions from dental staff [26]. It has been suggested that the dental team should be better educated and prepared for the needs of the patients with special needs like the autistic group [27]. Pilebro and Bäckman in 2005 proposed improvement in oral hygiene through visual pedagogy as a useful tool for people with autism [28]. Another possible explanation could be the side effects of medications used to control the manifestations of autism such as psychotic drugs or anticonvulsants [29]. A novel method known as Sensory Adapted Dental Environment (SADE) to reduce distress, sensory discomfort, and perception of pain during dental procedures has been proposed for autistic children [30].
Higher PI in the present study for the autistic group may indicate a higher risk of oral disease particularly dental caries. The oral condition of children with ASD might increase the risk of developing dental diseases [31]. In one study, although autistic group showed improvement in oral hygiene with a plaque control programme; yet that improvement was significantly higher among co-operative patients [32]. Their behaviour and life factors may complicate provision of services and limit access to dental care [31]. In a study, it was found that autistic children exhibited a higher caries prevalence, poor oral hygiene and wide-ranging unmet dental health needs than normal healthy control group [26]. In another study children with ASD were found to have lower DMFT scores and be more likely caries-free. However, it was found that they have higher unmet periodontal treatment needs than the normal control children [33]. In another study, similar dental caries status was observed in children with autism and their healthy normal siblings [24]. Shah et al., in 2015 also found that there was a higher complexity for periodontal treatment need for Down’s syndrome and behavioural disorders [34]. Apart from this, one study in Yemen about autistic children also proposed high prevalence of oral soft tissue lesions, caries, and gingivitis among the autistic group [35]. In this study, the mean Gingival Index (GI) and mean PI scores were slightly lesser than the scores in the present study. It is noteworthy that in the present study, MGI was used instead of the GI. The MGI has scores between 0-4 as compared to 0-3 for GI [18]. Regarding the PI it may be assumed to be either a variation in diet or plaque control measures such as tooth brushing.
Some studies have compared salivary parameters among autistic and non-autistic children [23,24,36]. Saliva is an important component in determining progression or inhibition of dental diseases [24]. In the present study, the pH of saliva of autistic group was slightly lesser than the other control group. This is in contrast to findings of Bassoukou et al., in 2009 [23]. However, this slight variation may be attributed to a small size of the sample as well as the dietary habits of the population. Salivary pH is very important particularly for dental caries as low pH can result in rapid demineralization of the enamel and thus aid in progression of dental caries [24]. In the present study there was no significant difference of buffering capacity of the stimulated saliva of the either groups. The buffering capacity of the saliva is a protective factor for the teeth with respect to the attack from the acidic environment in the mouth created by diet as well as the cariogenic bacteria [23].
Limitation
The limitations of this study are a limited sample size and a single centre study, it can be said that the oral cavity is a dynamic environment with many factors both present inside the saliva or from outside like the diet that can contribute to progression or regression of oral disease. To be able to recommend a certain oral health regime other factors associated with the oral health environment need to be examined. However, such studies may enable comparison and future planning of dental services to the autistic children and young adults.
Conclusion
Children with autism appear to have a higher gingival inflammation, poor oral hygiene and slightly lesser salivary pH when compared to healthy control group. Children with autism thus may be at higher risk of developing dental diseases. As such, special oral health program regarding treatment and maintenance of good oral health should be taken into consideration for autistic patients.
* Significant at p < 0.05
$ Not significant p > 0.05
[1]. Lord C, Cook EH, Leventhal BL, Amaral DG, Autism spectrum disordersNeuron 2000 28(2):355-63. [Google Scholar]
[2]. Newschaffer CJ, Croen LA, Daniels J, Giarelli E, Grether JK, Levy SE, The epidemiology of autism spectrum disorders*Annu Rev Public Health 2007 28:235-58. [Google Scholar]
[3]. Baio J, Prevalence of autism spectrum disorders: Autism and developmental disabilities monitoring network, 14 sites, United States, 2008. Centers of Disease Control and Prevention, Morbidity and Mortatlity Weekly ReportSurveillance Summaries 2012 61:1-19. [Google Scholar]
[4]. Boulet SL, Boyle CA, Schieve LA, Health care use and health and functional impact of developmental disabilities among US children, 1997-2005Arch Pediatr Adolesc Med 2009 163(1):19-26. [Google Scholar]
[5]. Elsabbagh M, Divan G, Koh YJ, Kim YS, Kauchali S, Marcin C, Global prevalence of autism and other pervasive developmental disordersAutism Res 2012 5(3):160-79. [Google Scholar]
[6]. Belmonte MK, Allen G, Beckel-Mitchener A, Boulanger LM, Carper RA, Webb SJ, Autism and abnormal development of brain connectivityJ Neurosci 2004 24(42):9228-31. [Google Scholar]
[7]. Loo CY, Graham RM, Hughes CV, The caries experience and behavior of dental patients with autism spectrum disorderJ Am Dent Assoc 2008 139(11):1518-24. [Google Scholar]
[8]. Kopycka-Kedzierawski DT, Auinger P, Dental needs and status of autistic children: results from the National Survey of Children’s HealthPediatr Dent 2008 30(1):54-58. [Google Scholar]
[9]. Klein U, Nowak AJ, Characteristics of patients with autistic disorder (AD) presenting for dental treatment: a survey and chart reviewSpec Care Dentist 1999 19(5):200-07. [Google Scholar]
[10]. Stein LI, Polido JC, Mailloux Z, Coleman GG, Cermak SA, Oral care and sensory sensitivities in children with autism spectrum disordersSpec Care Dentist 2011 31(3):102-10. [Google Scholar]
[11]. Stein LI, Polido JC, Najera SOL, Cermak SA, Oral care experiences and challenges in children with autism spectrum disordersPediatr Dent 2012 34(5):387-91. [Google Scholar]
[12]. DeMattei R, Cuvo A, Maurizio S, Oral assessment of children with an autism spectrum disorderJ Dent Hyg 2007 81(3):65 [Google Scholar]
[13]. Shapira J, Mann J, Tamari I, Mester R, Knobler H, Yoeli Y, Oral health status and dental needs of an autistic population of children and young adultsSpec Care Dentist 1989 9(2):38-41. [Google Scholar]
[14]. Marshall J, Sheller B, Mancl L, Caries-risk assessment and caries status of children with autismPediatr Dent 2010 32(1):69-75. [Google Scholar]
[15]. Lai B, Milano M, Roberts MW, Hooper SR, Unmet dental needs and barriers to dental care among children with autism spectrum disordersJ Autism Dev Disord 2012 42(7):1294-303. [Google Scholar]
[16]. Delli K, Reichart PA, Bornstein MM, Livas C, Management of children with autism spectrum disorder in the dental setting: concerns, behavioural approaches and recommendationsMed Oral Patol Oral Cir Bucal 2013 18(6):e862-68. [Google Scholar]
[17]. Lobene RR, Weatherford T, Ross NM, Lamm RA, Menaker L, A modified gingival index for use in clinical trialsClin Prev Dent 1986 8(1):3-6. [Google Scholar]
[18]. Rebelo MAB, De Queiroz AC, Gingival indices: state of art 2011 INTECH Open Access Publisher [Google Scholar]
[19]. Feier I, Onisei D, Onisei D, The plurivalence of the interpretation of correlation between plaque score and bleeding scoreJournal of Romanian Medical Dentistry 2009 13(1):45-48. [Google Scholar]
[20]. Almasoud H, Services and support for individuals with autism: A comparative study between the UK and Saudi Arabia King Saud University Document 2010 :1-17. [Google Scholar]
[21]. Dalton AJ, McVilly KR, Ethics guidelines for international, multicenter research involving people with intellectual disabilities1, 2, 3, 4Journal of Policy and Practice in Intellectual Disabilities 2004 1(2):57-70. [Google Scholar]
[22]. Baron-Cohen S, Scott FJ, Allison C, Williams J, Bolton P, Matthews FE, Prevalence of autism-spectrum conditions: UK school-based population studyBr J Psychiatry 2009 194(6):500-09. [Google Scholar]
[23]. Bassoukou IH, Nicolau J, dos Santos MT, Saliva flow rate, buffer capacity, and pH of autistic individualsClin Oral Investig 2009 13(1):23-27. [Google Scholar]
[24]. Rai K, Hegde AM, Jose N, Salivary antioxidants and oral health in children with autismArch Oral Biol 2012 57(8):1116-20. [Google Scholar]
[25]. Vishnu Rekha C, Arangannal P, Shahed H, Oral health status of children with autistic disorder in ChennaiEur Arch Paediatr Dent 2012 13(3):126-31. [Google Scholar]
[26]. Jaber MA, Dental caries experience, oral health status and treatment needs of dental patients with autismJ Appl Oral Sci 2011 19(3):212-17. [Google Scholar]
[27]. Shah AH, Fateel A, Al-Nakhli O, Dentists and dental students opinion regarding dental treatment of patients with special needsJPDA 2011 20(2):98-104. [Google Scholar]
[28]. Pilebro C, Backman B, Teaching oral hygiene to children with autismInt J Paediatr Dent 2005 15(1):1-9. [Google Scholar]
[29]. Nagendra J, Jayachandra S, Autism spectrum disorders: Dental treatment considerationsJ Int Dent Med Res 2012 5(2):118-21. [Google Scholar]
[30]. Cermak SA, Stein Duker LI, Williams ME, Dawson ME, Lane CJ, Polido JC, Sensory adapted dental environments to enhance oral care for children with autism spectrum disorders: a randomized controlled pilot studyJ Autism Dev Disord 2015 45(9):2876-88. [Google Scholar]
[31]. El Khatib AA, El Tekeya MM, El Tantawi MA, Omar T, Oral health status and behaviours of children with autism spectrum disorder: A case-control studyInt J Paediatr Dent 2014 24(4):314-23. [Google Scholar]
[32]. Dias GG, Prado EF, Vadasz E, Siqueira JT, Evaluation of the efficacy of a dental plaque control program in autistic patientsJ Autism Dev Disord 2010 40(6):704-08. [Google Scholar]
[33]. Fakroon S, Arheiam A, Omar S, Dental caries experience and periodontal treatment needs of children with autistic spectrum disorderEur Arch Paediatr Dent 2015 16(2):205-09. [Google Scholar]
[34]. Shah A, Bindayel N, AlOlaywi F, Sheehan S, AlQahtani H, AlShalwi A, Oral health status of a group at a special needs centre in AlKharj, Saudi ArabiaJournal of Disability and Oral Health 2015 16(3):79-85. [Google Scholar]
[35]. Al-Maweri SA, Halboub ES, Al-Soneidar WA, Al-Sufyani GA, Oral lesions and dental status of autistic children in Yemen: A case-control studyJ Int Soc Prev Community Dent 2014 4(Suppl 3):199-203. [Google Scholar]
[36]. Ngounou Wetie AG, Wormwood KL, Russell S, Ryan JP, Darie CC, Woods AG, A pilot proteomic analysis of salivary biomarkers in autism spectrum disorderAutism Res 2015 8(3):338-50. [Google Scholar]