Tuberculosis remains a challenge in Iran, and the annual incidence was reported as 22 per 100,000 people in 2014 [1]. The main genotype families of Mycobacterium tuberculosis include the Beijing, Haarlem, Africa, East-African-Indian, Latin American Mediterranean (LAM), and Mediterranean genotypes. From this family, the Beijing genotype has some selective advantages [2].
Pathogenic features of these genotypes are important, such as the ability to rapidly proliferate in human macrophages, high transmissibility, escape from BCG vaccination, and association with multi-drug resistance [3]. The Beijing genotype appears to have spread globally and is also associated with several outbreaks [4]. It appears to be endemic in Russia, South Africa, and East Asia. It can also be found less frequently in Africa as a whole, the United States, and Europe [5]. Furthermore, some valuable studies showed that the Beijing genotype is associated with increased frequency of drug resistance compared with non-Beijing strains [6]. There are various molecular methods for typing of M. tuberculosis and detection of the Beijing genotype. Spoligotyping is the recommended method for genotyping. This method is expensive, time-consuming to use, and requires special equipment [7]. Furthermore, another method is IS6110 DNA fingerprinting, which requires technical expertise and is time-consuming [8]. Compared with other methods, multiplex PCR is rapid, easy, and inexpensive, and gives reliable results [7]. This method can detect several genes in one reaction. Thus, Beijing and non-Beijing infection and mixed infections could also be diagnosed through the detection of their corresponding genes.
Considering the importance of the Beijing genotype and its outcome, this study was conducted to identify Beijing and non-Beijing genotypes simultaneously by multiplex PCR. In addition, the association between drug resistance and Beijing genotype was analysed.
Materials and Methods
This study was a cross-sectional study conducted from January 2014 to February 2015 at the TB reference laboratory of Kermanshah, western Iran. From 5 provinces (Kermanshah, Lorestan, Hamedan, Ilam, and Kurdistan), isolates of M. tuberculosis were collected. The mean age of the patients was 54 ± 20 years. Patients were divided in 2 groups based on their ages, 17–50 and ≥51. Eighty (55%) of them were male and 66 (45%) were female.
A total of 523 sputum samples suspected of having tuberculosis were collected and stained by the Auramine and Ziehl-Neelsen methods after decontamination [9]. Then from these, 210 smear positive samples were cultured on Lowenstein-Jensen (LJ) medium and biochemical tests such as nitrate reduction, niacin accumulation, catalase activity at 68°C, and pigment production [10] for further identification. A total of 156 culture positive samples were confirmed by Cepheid Xpert MTB/RIF assay G4 version 5. Gen Xpert confirmed 146 samples of M. tuberculosis and these samples were further processed for antibiotic susceptibility and PCR.
Antibiotic Susceptibility Test
Positive samples from geneXpert were taken for drug susceptibility testing including the following first-line drugs: rifampicin (40.0μg/mL), isoniazid (0.2μg/mL), and ethambutol (2.0μg/mL), performed using the proportional method following the current recommendations from the World Health Organization (WHO) [9]. The standard criteria for diagnosing resistant bacteria based on the proportional method is the percentage of colonies that can grow on drug-containing medium compared with colonies that typically grow on drug-free medium (growth of ≥1% of colonies) [9].
PCR
The bacterial suspension from colonies grown on LJ agar was placed in a boiling water bath for 90 min to destroy any viable mycobacteria and bacterial cell walls. Firstly, the bacterial suspension was centrifuged at 5000 rpm for 10 min, then the supernatant was transferred to a new tube. Secondly, bacterial DNA was extracted by centrifugation at 14000 rpm for 30 min. The sediment was used as a DNA template.
All primers in this study were based on a previous study by Weng [11].
In brief, first set primers to distinguish M. tuberculosis, IS60 (GATCAGCGATCGTGGTCCTGC) and IS59 (GCGCCAGGCG CAGGTCGATGC), were used to amplify a 523-bp PCR product, to serve as the PCR control. Two sets of primers were used to detect the Beijing and non-Beijing genotypes: BjF (CTCGGCAGCTTCCTCGAT) and BjR (CGAACTCGAGGCTGC CTACTAC) produced a 129-bp PCR product, and nBjF (AAGCATTCCCTTGACAGTCGAA) and nBjR (GGCGCATGACTCGAAA GAAG), produced a 104-bp PCR product.
PCR was performed in a total volume of 25μL, containing 2.5μM dNTP mix, 2.0mM MgCl2, 0.5U Taq DNA polymerase, 0.5μM of each primer, and 2.5μl each of template DNA and10× buffer.
Cycling conditions were as follows: 96°C for 5 min, followed by 35 cycles of 94°C for 30s, 58°C for 30s, and 72°C for 1min. A final extension at 72°C for 7min was also performed and PCR products were separated on 15% acrylamide gels.
A positive control for the Beijing genotype was retrieved from Tehran University of Medical Science [12]. H37Rv DNA of the Center for Tuberculosis-related Disease was used as a positive control for the non-Beijing genotype.
DNA Sequencing
To confirm our results, one PCR product each of the Beijing and non-Beijing genotypes was sent for sequencing (Bioneer co). The Beijing genotype was based on GenBank accession no.cp0010873.1.
Statistical Analysis
Statistical analysis was performed with SPSS software version 16. All of the data was analysed using a chi-square or Fisher-exact test (p<0.05).
Results
Multiplex PCR showed 15 Beijing and 131 non-Beijing geno-types.
The Beijing genotype gave expected PCR product sizes of 523 bp and 129 bp. A non-Beijing genotype showed PCR products of 523 bp and 104 bp. Mixed infection samples were expected to yield all PCR product sizes: 523 bp, 104 bp, and 129 bp.
The majority of the sputum samples (14 of 15) of Beijing cases showed mixed infection, indicating the presence of both Beijing and non-Beijing strains in the samples [Table/Fig-1]. From Kermanshah province maximum positive samples of MTB were isolated [Table/Fig-2].
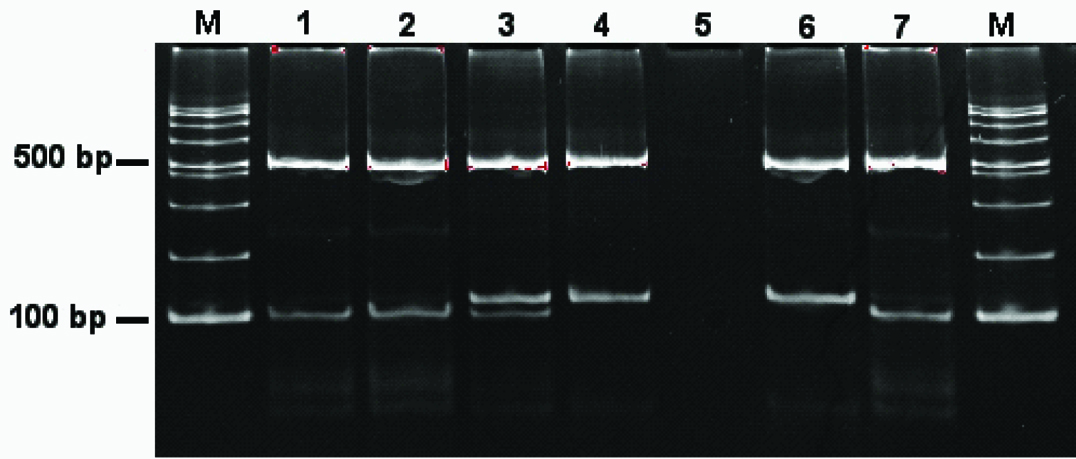
Electrophoresis results of the multiplex PCR products on a 15% polyacrylamide gel. Lane M, 100-bp ladder DNA size marker; lane 1-4 M. tuberculosis clinical isolates (lane 1 and 2, non-Beijing; lane 3, mix infection; lane 4, Beijing); lane5, negative control; lane 6, M. tuberculosis H37Rv (non-Beijing genotype control); lane 7, M. tuberculosis H-17 strain (Beijing genotype control); lane M contains molecular size markers (100-bp ladder vivantis).
Prevalence of Beijing genotype in this study.
| No. | Province | Positive sample of MTB | Beijing genotype |
|---|
| 1 | Kermanshah | 40 | 6 |
| 2 | Kurdistan | 39 | 1 |
| 3 | Lorestan | 31 | 4 |
| 4 | Ilam | 16 | 2 |
| 5 | Hamadan | 20 | 2 |
| Total | 146 | 15 |
|---|
Only 3 patients were associated with drug resistant strains, 2 of them were resistant to rifampicin and the other isolate was simultaneously resistant to rifampicin, isoniazid, and ethambutol.
More than 90% of the clinical isolates were new cases who had not received anti-Tuberculosis (TB) drugs. All drug resistant isolates were from new cases. The Beijing association with newly diagnosed drug resistance cases showed significant increases in rifampicin, isoniazid, and ethambutol (p≤0.001).
Of the total 146 cases, 60 cases were <50-year-old and 86 cases were >50-year-old, of which 11 (18.3%) and 4 (4.6%) of them were patients infected with Beijing and non-Beijing genotypes, respectively.
Considering this data, a significant correlation between younger age group and the Beijing genotype was found (p=0.035).
Discussion
The Beijing genotype has received attention because of its prevalence in different areas [2]. Our study showed with multiplex PCR assay, that in 5 provinces, among 146 PCR-confirmed MTB isolates, 15 (10.4%) isolates detected were of the Beijing genotype. Beijing genotypes appeared to have different distributions according to geographic region, with a high distribution in China and Russia [2]. The Beijing genotype was also detected in Iran [2]. Based on previous studies in the Eastern, Southwestern, and capital regions, the rates of Beijing genotypes were 7.1%, 5%, and 9.5%, respectively [4,13,14]. Although the frequency of the Beijing genotype in Western Iran is higher than in other areas of the country, in comparison with other Asian countries, it has less prevalence [15].
In this study, the frequency of the Beijing genotype based on age was 73% in <50-year-old. Studies show a high prevalence of the Beijing genotype in younger TB patients in Western Europe, Southern Africa, and the former Soviet Union [6], despite the fact that in São Paulo and Buenos Aires, patients <30-year-old were infected by all Beijing genotype [16]. There is a little evidence of a high prevalence of the Beijing genotype in younger patients in Asia, except for Vietnam and Bangladesh [6]. The correlation between the Beijing genotype and drug resistance is concerning [2]. This study conducted in Western Iran showed a strong association between drug resistance and the Beijing genotype. The results of studies carried out in Vietnam, Bangladesh, Taiwan, Hong Kong, other Asian countries, and many European countries are inline with these results [16]. The distribution of mixed infection in TB patients varies among studies [17,18]. The prevalence of the Beijing and non-Beijing genotypes (mix infections) among patients was 93%. A study from South Africa showed that 57% of patients infected with the Beijing genotype were also infected with the non-Beijing genotype [19]. Mixed infections including Beijing and non-Beijing genotypes may contribute to an increased risk of MDR [20]. Moreover, the presence of simultaneous infection can lead to a disturbance in the outcome of drug susceptibility testing if both, drug-susceptible and resistant M. tuberculosis strains were present [18].
Detecting mixed infection with M. tuberculosis strains may be very difficult [21]. Spoligotyping is the gold standard for the detection and classification of the Beijing genotype [7]. It can be used to diagnose the Beijing genotype but it cannot detect mix infections [22]. This technique also needs special equipment and is time consuming and somewhat expensive. A multiplex PCR method can rapidly detect Beijing and non-Beijing strains [7].
Limitation
In this study, one of the major limitation was inadequate demographic data about patients from different provinces. Data on multiplex PCR method and other molecular methods are needed for selection of best method in the diagnosis of Beijing and non-Beijing genotypes, which could not be collected in this study.
Conclusion
This study showed that the Beijing genotype of M. tuberculosis is an epidemic in our province. We also found a significant correlation between younger age group and the Beijing genotype. Multiplex PCR is an inexpensive and easy to perform method; the important advantage of multiplex PCR in contrast to spoligotyping is its utility in the diagnosis of mix infections with Beijing and non-Beijing genotypes. Considering the results of our study, mixed infection is prevalent; therefore, we should prefer to perform multiplex PCR in addition to spoligotyping to recognize mixed infection. However, to save time, it seems that the multiplex PCR method is an inexpensive and simple technique for use in areas of high prevalence of Beijing strains in some parts of the world.