Traumatic Ulcerative Granuloma with Stromal Eosinophila: A Case Report and Review of Pathogenesis
Bhushan Sharma1, George Koshy2, Shekhar Kapoor3
1 Associate Professor, Department of Oral Pathology, Christian Dental College, C.M.C., Ludhiana, Punjab, India.
2 Professor, Department of Oral Pathology, Christian Dental College, C.M.C., Ludhiana, Punjab, India.
3 Associate Professor, Department of Oral Medicine and Radiology, Christian Dental College, C.M.C., Ludhiana, Punjab, India.
NAME, ADDRESS, E-MAIL ID OF THE CORRESPONDING AUTHOR: Dr. Bhushan Sharma, Associate Professor, Department of Oral Pathology, Christian Dental College, C.M.C., Ludhiana, Punjab, India.
E-mail: bhushan_1707@yahoo.co.in
Traumatic Ulcerative Granuloma with Stromal Eosinophilia (TUGSE) is an uncommon condition considered to be a, reactive benign lesion of the oral mucosa, usually affecting the tongue. Its aetiopathogenesis is still uncertain. However, trauma has been found to be a contributing factor in a majority of the cases. Clinically, it often presents as an isolated ulcer or an indurated submucosal mass. Microscopically, it is characterized by a diffuse polymorphic cell infiltrate composed predominantly of eosinophils, B and T lymphocytes, macrophages, and large atypical cells involving the superficial mucosa and extending deep into the submucosa causing degeneration of the underlying muscle. TUGSE is rare and may be easily mistaken for a cancer or microbial infection, but it is self-limiting and tends to resolve spontaneously. Thus, awareness of this entity is important to emphasize the correct diagnosis of indurated ulcerated lesions and deliver appropriate and effective treatment. The present case highlights the clinical aspects, aetiopathogenesis and histopathology of this uncommon lesion.
Eosinophils, Reactive benign lesion, Riga-fede disease, Tongue
Case Report
A 75-year-old woman presented with a chief complaint of painful ulcer on the left side of the postero-lateral aspect of tongue since 20 days. She gave history of pain being moderate, continuous and radiating to cheek and neck region. There was no relief after medication. Patient did not give any history of local trauma by adjacent teeth. On clinical examination, an ulcer was seen on the left lateral border of tongue, opposite teeth 35 and 36. Close inspection revealed an erythematous ulcer of 1cm diameter with a whitish surrounding halo. On palpation the ulcer was smooth, tender and firm in consistency, with well defined margins and induration. There was no fixation to the deeper structures or any regional lymphadenopathy. Her medical history was unremarkable. Provisional diagnosis of traumatic ulcer was made. An excisional biopsy was performed under local anesthesia for histopathological examination. Microscopic examination showed superficial hyperplastic epithelium displaying hyperkeratosis with a central area of ulceration [Table/Fig-1]. Superficial epithelial cells exhibited mucoplysaccharide keratin dystrophy [Table/Fig-2]. The area of ulceration was infiltrated with dense mixed inflammatory cells chiefly composed of eosinophils and macrophages [Table/Fig-3]. These cells extend deep into the musculature of the tongue with evidence of infiltrating the muscle fibers. The infiltrated tissue was well vascularized. No atypical cells were seen. Based on the clinico-pathologic features, a diagnosis of Traumatic Ulcerative Granuloma with Stromal Eosinophilia (TUGSE) was arrived. Complete healing was noticed after 1 month. No treatment was required other than regular observation and routine check-ups.
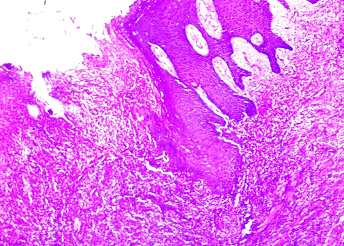
Ulcerated epithelium and connective tissue infiltrated with mononuclear inflammatory cell infiltrate (4X, H&E).
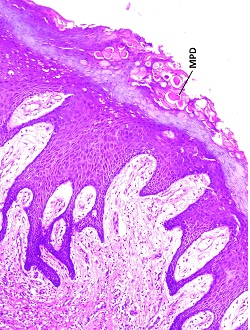
Superficial epithelial cells exhibited mucoplysaccharide keratin dystrophy (MPD) (10X, H&E).
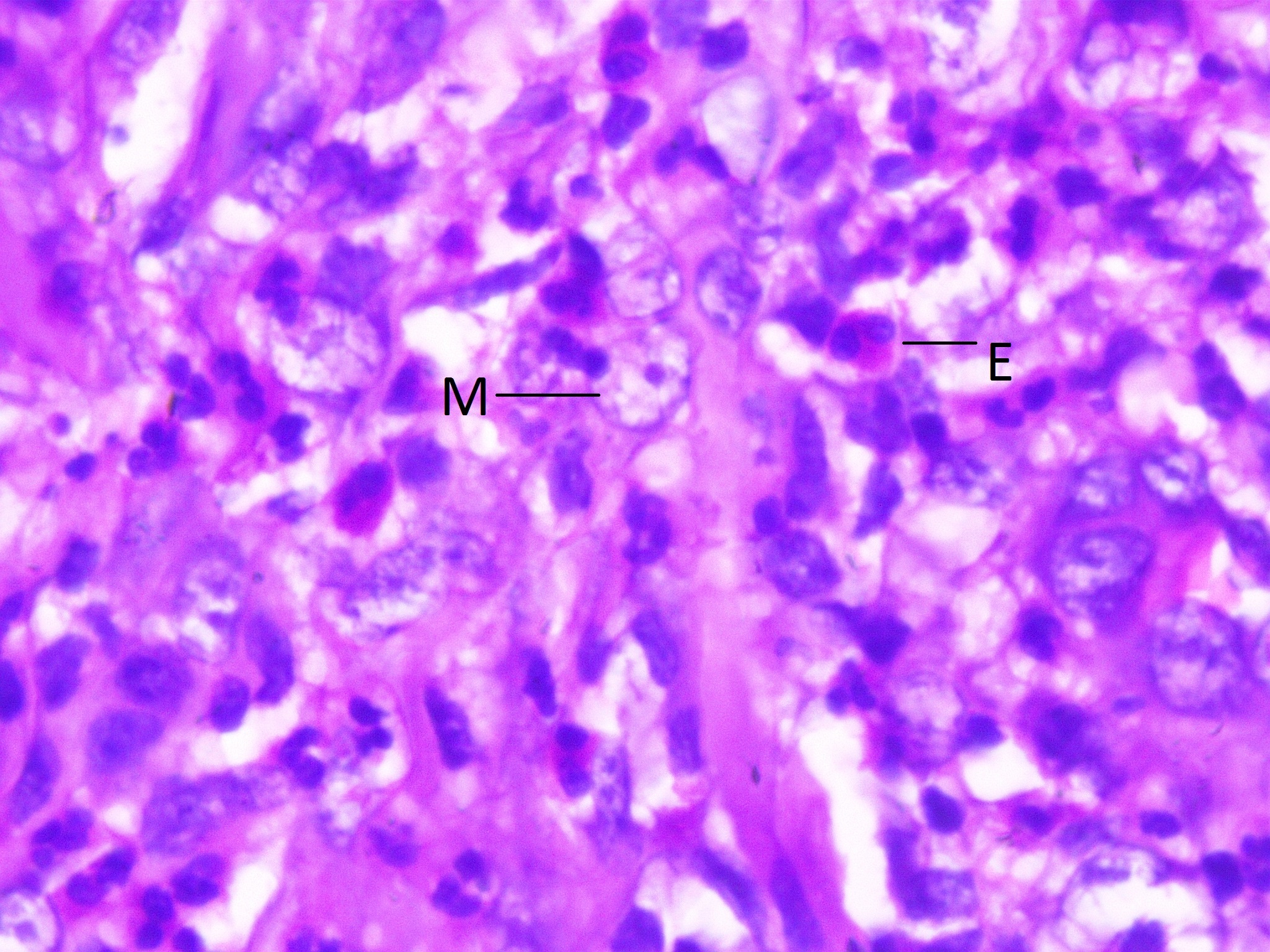
Eosinophils (E) and macrophages (M) seen in higher magnification (100X, H&E).
Discussion
TUGSE is a rare and unique lesion with uncertain nature, aetiology and pathogenesis. It is considered to be a benign, reactive and chronic but self-limiting reactive ulcer of the oral mucosa [1]. TUGSE was originally described clinically in 1881 by Riga and histologically in 1890 by Fede [1,2]. The term TUGSE was coined by Elzay in 1983 [3]. This lesion has been known by different names, Riga-Fede disease in infants and neonates, sublingual granuloma, traumatic granuloma, eosinophilic granuloma, eosinophilic ulcer, and ulcerative eosinophilic granuloma [4,5]. In 1970, this lesion was proposed as a distinct entity by Shapiro and Juhlin [6].
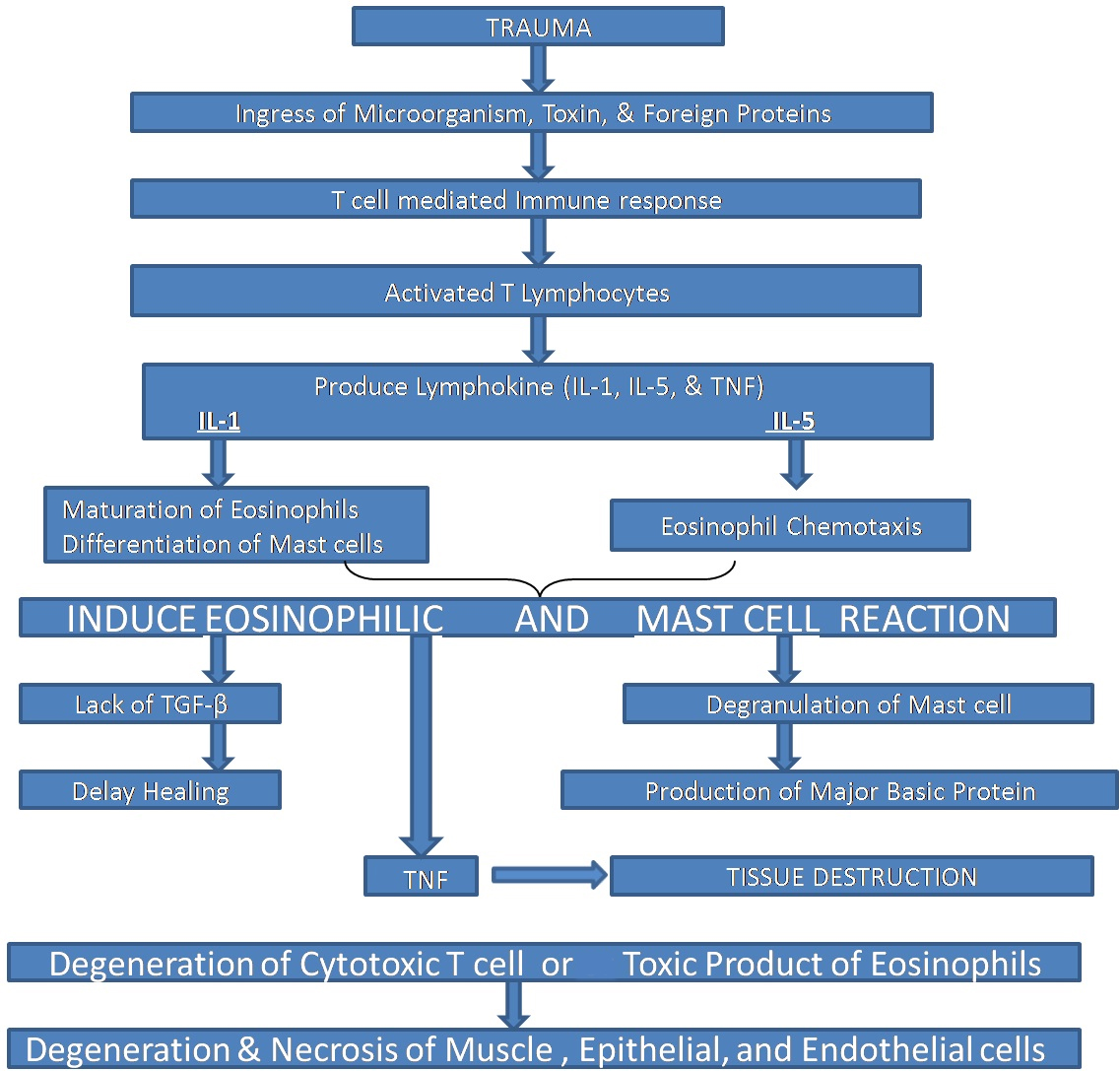
The aetiology of TUGSE remains obscure but the main theory is matched with trauma; however, injury is identified in less than 50% of cases [7]. Viral or toxic agent hypothesis [6,8,9] and accidental bites [10] have also been included in its development. Experimental studies with rats demonstrated similar lesions after repeated injury to the tongue [4]. Others have suggested that recurrent trauma leads to alteration of tissue antigens or introduces toxins, micro-organisms, endogenous degradation products or foreign proteins [10,11] into the tissues; either event could incite a local immune reaction [12]. Some authors have even reported a possible interaction among mast cells, release of eosinophil chemotactic factors and tissue eosinophilia [13]. However, an absolute linkage between trauma and the development of such lesions wasn’t found in all studies [14,15]. Due to a variety of cells found in inflammatory infiltrate TUGSE is thought to be a benign, reactive lesion. El-Mofty et al., concluded that cell-mediated immunity might play an important role in the pathogenesis [12]. In brief we have explained the pathogenesis of TUGSE in flowchart [Table/Fig-4].
Depicting pathogenesis of TUGSE.
Clinically, most of them appear as a rapidly developing solitary ulcer with elevated or indurated margins [15,16]. In addition, sub-mucosal masses, multifocal lesions and recurrences have also been mentioned in the literature [17,18]. It may be symptomatic associated with mild to severe pain in many cases, suggestive of malignancy and infections such as deep fungal infections, tuberculosis and primary syphilis [19] and this is where the condition becomes important. It can persist for several weeks or months and heals without any treatment. A wide age range of patients are affected, seen from childhood to old age with a peak incidence between the sixth and seventh decades of life [8,10]. TUGSE exhibits a slight female predominance in most of the cases. It occurs mainly on the dorsal or lateral surface of the tongue (60% of lesions), which seems reasonable since movement makes it more vulnerable to trauma [6,10,12]. Although other areas such as lip, palate, gingiva, vestibular mucosa and floor of the mouth may also be involved [18,20]. Clinically indurated lesions represent an earlier phase in the development of the typical ulcer [12]. However, the microscopic studies and immunohistochemical features indicate a benign process. Lymphadeonapathy can be observed in extremely rare cases [21].
In our case, the TUGSE was present on the left postero-lateral surface of the tongue measuring about 1cm in diameter, with a pale surrounding area. Its margins were indurated and tender on palpation. It occurred in a female patient, in the seventh decade of life, and presented without lymphadeonapathy. The clinical differential diagnoses may include traumatic neuroma, lymphoma, lymphangioma, salivary gland tumors, primary syphilis, metastasis, atypical histiocytic granuloma and proliferative myositis. Histologically, TUGSE show a diffuse polymorphic inflammatory infiltrate predominantly composed of eosinophils and histiocytes, accompanied by a population of large mononuclear T and B cells, and macrophages [8,10] with abundant cytoplasm, irregular nuclear contours, small nucleoli and fine chromatin [13]. It involves the superficial mucosa and extending deep into the submucosa involving the muscle and even salivary glands. Some cases have shown a pseudolymphomatous appearance [9]. The similar histopathological features were observed in present case. The histologic differential diagnosis may include reactionary proliferative processes such as atypical histiocytic granuloma, Langerhan’s cell histiocytosis, proliferative myositis, Angiolymphoid Hyperplasia with Eosinophilia (ALHE), Kimura disease, certain types of lymphomas, allergic reactions and parasitic diseases [22,23].
Immunohistochemical characteristic has been a matter of debate because of the unknown origin of the large, atypical, mononuclear cells. Some authors have reported the origin from macrophages (CD68-positive) [6] dendritic cells (Factor XIII-positive) [11] and even some from myofibroblasts (vimentin-positive) [11], so they might play a role in the reparative phase of the lesion. The latest survey reveals that these are CD30 positive cells originating from T lymphocytes [22]. CD30 is a histological marker for lymphoproliferative disease, Hodgkin’s lymphoma and RS cells. However, it also occur in many non-neoplastic cutaneous disorders such as atopic dermatitis, adverse drug reaction, molluscum contagiosum and insects and spiders bites [10,11]. Ficarra et al., first hypothesized a possible lymphoproliferative origin on the basis of clinical and histological findings [6]. Alobeid et al., supported this feature by immunohistochemical and PCR analysis [24]. Segura and Pujol reviewed eosinophilic ulcer of the oral mucosa and considered it as a non-specific reactive pattern rather than a distinct entity [10].
Several therapeutic approaches like topical steroids, mouthwashes, topical antibiotics, curettage and cryotherapy have been reported [8,9]. The most frequently performed therapy is simple surgical excision. No local recurrences are usually noted after excision [11,13,23]. In our case simple excision was performed and a rapid improvement of the ulcer was seen similar to what was described by other authors [9,10].
Conclusion
In conclusion, diagnosis of TUGSE is made by the combination of clinical and histopathological features. The pathogenesis of this condition remains uncertain and its histogenesis still remains controversial and this condition is characteristically self-healing with a benign course.
[1]. Riga A, Di una malattia della prima infanzia, Probabilmente non-trattata, di movimenti patologiciNapoli 1881 [Google Scholar]
[2]. Fede F, Della produzione sottolinguale o malattia di Riga. Atto Congresso italiano di pediatria 1890Napoli 1891 251 [Google Scholar]
[3]. Elzay RP, Traumatic ulcerative granuloma with stromal eosinophilia (Riga-Fede’s disease and traumatic eosinophilic granuloma)Oral Surg Oral Med Oral Pathol 1983 55:497-506. [Google Scholar]
[4]. Welborn JF, Eosinophilic granuloma of tongue: Report of a caseJ Oral Surg 1966 24:176-79. [Google Scholar]
[5]. Tang TT, Glicklich M, Hodach AE, Oechler HW, McCreadie SR, Ulcerative eosinophilic granuloma of the tongueAm J Clin Pathol 1981 75:420-25. [Google Scholar]
[6]. Ficarra G, Prignano F, Romagnoli P, Traumatic eosinophilic granu- loma of the oral mucosa: A CD30+(Ki-1) lymphoproliferative dis-order ?Oral Oncol 1997 33(5):375-79. [Google Scholar]
[7]. Segura S, Romero D, Colomo L, Eosinophilic ulcer of the oral mucosa: Another rhistological simulator of CD30+ lymphoproliferative disordersBrit J Dermatol 2006 155:460-63. [Google Scholar]
[8]. Gao S, Wang Y, Liu N, Li S, Du J, Eosinophilic ulcer of the oral mucosa: A clinicopathological analysisChin I Dent Res 2000 3(1):47-50. [Google Scholar]
[9]. Boffano P, Gallesio C, Campisi P, Roccia F, Traumatic ulcerative granuloma with stromal eosinophilia of the retromolar regionJ Craniofac Surg 2009 20(6):2150-52. [Google Scholar]
[10]. Segura S, Pujol RM, Eosinophilic ulcer of the oral mucosa: A distinct entity or a non-specific reactive pattern?Oral Dis 2008 14(4):287-95. [Google Scholar]
[11]. Hirshberg A, Amariglio N, Akrish S, Yahalom R, Rosenbaum H, Okon E, Traumatic ulcerative granuloma with stromal eosynophilia: Reactive lesion of the oral mucosaAm J Clin Pathol 2006 126:522-29. [Google Scholar]
[12]. El-Mofty SK, Swanson PE, Wick MR, Miller AS, Eosinophilic ulcer of the oral mucosa: Report of 38 new cases with immunohistochemical observationsOral Surg Oral Med Oral Pathol 1993 75:716-22. [Google Scholar]
[13]. Gonçales ES, Rubira-Bullen IF, Rubira CM, Miyazawa M, Chinellato LE, Consolaro A, Eosinophilic ulcer of the oral mucosa versus squamous cell carcinomaQuintessence Int 2007 38(8):677-80. [Google Scholar]
[14]. Elovic AE, Gallgher GT, Kabani S, Galli SJ, Weller PF, Wang DT, Lack of TGF-a and TGF-b synthesis by human eosinophils in chronic oral ulcersOral Surg Oral Med Oral Pathol Oral Radiol Endod 1996 81:672-81. [Google Scholar]
[15]. Vélez A, Alamillos FJ, Dean A, Rodas J, Acosta A, Eosinophilic ulcer of the oral mucosa: Report of a recurrent case on the tongueClin Exp Dermatol 1997 22(3):154-56. [Google Scholar]
[16]. Doyle JL, Geary W, Baden E, Eosinophilic ulcerJ Oral Maxillofac Surg 1989 47:349-52. [Google Scholar]
[17]. Velez A, Alamillos FJ, Dean A, Rodas J, Acosta A, Eosinophilic ulcer of the oral mucosa: Report of a recurrent case on the tongueClin Exp Dermatol 1997 24:154-56. [Google Scholar]
[18]. Sklavonou A, Laskaris G, Eosinophilic ulcer of the oral mucosaOral Surg Oral Med Oral Pathol 1984 58:431-36. [Google Scholar]
[19]. Bouquot JE, Muller S, Nikai H, Lesions of the oral cavity In: GNEP DR, edsDiagnostic Surgical Pathology of the Head and Neck 2009 PhiladelphiaSaunders:267-77. [Google Scholar]
[20]. Pilolli GP, Lucchese A, Scivetti M, Maiorano E, Favia G, Traumatic ulcerative granuloma with stromal eosinophilia of the oral mucosa: Histological and immunohistochemical analysis of three casesMinerva Stomatol 2007 56:73-79. [Google Scholar]
[21]. Salisbury CL, Budnick SD, Li S, T cell receptor gene rearrangement and CD 30 immunoreactivity in traumatic ulcerative granuloma with stromal eosinophilia of oral cavityAm J Clin Pathol 2009 132:722-27. [Google Scholar]
[22]. Cepeda LT, Pieretti M, Chapman SF, Horenstein MG, CD30-positive atypical lymphoid cells in common on-neoplastic cutaneous infiltrates rich in neutrophils and eosinophilsAm J Surg Pathol 2003 27:912-18. [Google Scholar]
[23]. Ada S, Seckin D, Tarhan E, Buyuklu F, Cakmak O, Arikan U, Eosinophilic ulcer of the tongueAustralas J Dermatol 2007 48(4):248-50. [Google Scholar]
[24]. Alobeid B, Pan LX, Milligan L, Budel L, Frizzera G, Eosinophil-rich CD30+ lymphoproliferative disorder of the oral mucosa. A form of ‘traumatic eosinophilic granuloma’Am J Clin Pathol 2004 121:43-50. [Google Scholar]