Head and neck cancer continues to persist as a substantial public health disorder throughout the world. These account for up to 40% malignancies in Eastern countries including India. Despite advances in treatment modalities the survival rate did not meliorate over the past 20-30 years. Epidemiological studies also depict connection between alcohol, tobacco and arecanut consuming habits with the occurrence of malignant neoplasm of oral cavity. Such malignancy is due to alterations in frequency of epithelial cell proliferation which were observed in tobacco users [1–4]. National Family Health Survey (NFHS-3) was conducted in 2005–06 which reported that one third of the men and around one tenth of the woman use smokeless tobacco in India resulting in pre-malignant lesions [5]. Squamous cell carcinoma is the most common and has the highest incidence rate that originates as a Potentially Malignant Lesions (PML) in the oral cavity. However, the fate of these PMLs is undeterminable as some may revert back to normal whereas about one third may proceed to malignancy. Such numerous lesion in the oro-pharyngeal tract has given rise to the conception of field cancerization by Slaughter et al., in 1953, describing about the histologically abberant tissues encompassing the Oral Squamous Cell Carcinoma (OSCC) [6]. The consequences of carcinogenic insult to the oral tissues results in chromosomal damage in early cell divisions which can be examined in routine cytopathological smears as a micronuclei, before the development of clinical manifestations [7,8]. Hence, it would be of practical importance to develop biomarkers which will help in identifying the persons at greater risk of second field tumors and who have been treated for first primary tumor and stays at increased chances for generating second primary tumor [6,9,10].
A Mn is a tiny auxiliary nucleus detached from the major one, developed during cellular segregation by late chromosomes or chromosome particle [11]. An assesment of the literature shows that differences exist in Mn studies and attempts were made to standarize the test, but untill now not much effort is given to the effect of different staining methods [12,13]. Many studies have shown conflicting results about the reliability of the DNA specific and non-specific stains [14–18]. Hence, in this study, the genotoxic effects of tobacco habits using Mn assay and its correlation with other nuclear anomalies were evaluated as well as the reliability of DNA specific stain (feulgen) over the non-specific DNA stain (PAP) was checked.
Materials and Methods
Subjects: The present case-control study was conducted in the Department of Oral and Maxillofacial Pathology and Microbiology of Kanti Devi Dental College and Hospital, Mathura, Uttar Pradesh, India, from the year 2011 to 2013. The study group comprised of 90 individuals which were divided into three groups. A previous pilot study was conducted to determine the sample size and feasibility of the study in 60 subjects and the results of the pilot study were included in the present study. The power of study was taken at 80% and 20% type II error was allowed. Group 1 comprised of 30 individuals who were having tobacco habits (smoking and chewing) without lesion and Group 2 with 30 individuals who were having tobacco habits (smoking and chewing) with potentially malignant lesions (leukoplakia, erythroplakia and proliferative verrucous leukoplakia). Only leukoplakic lesions were included as it was the most commonly and frequently reported precancerous lesion in the department. Smokers who smoked every day for at least five years and smokeless tobacco chewers, who chewed daily for at least five years, were included in their respective groups.
Group 3 was the control group comprising of 30 apparently healthy subjects who had never smoked, consumed tobacco or arecanut in any form and had no visible mucosal lesion and had no history of viral disease or any medication during the preceding six months. They were subjected to clinical examination and cytosmear of the buccal mucosa were made [Table/Fig-1].
Cytosmear sampling from the buccal mucosa using cytobrush in the lesional group.
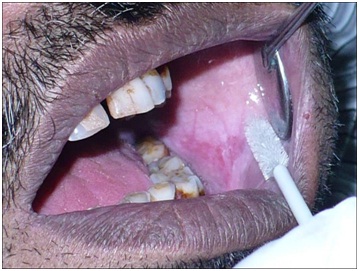
Clinical Procedure
Cytosmear: Subjects were asked to rinse their mouth thoroughly with water. Exfoliated buccal cells were obtained by rolling the cytobrush against the buccal mucosa for 1minute. Two smears were made from each subject.
Smear preparation: The cells were smeared over a pre cleaned coded microscopic slide and immediately fixed with Zenker’s fluid.
Staining procedure and Mn scoring: Fixed smear was subjected to PAP and feulgen staining. Staining was performed by the standard technique of Feulgen and Rossenback (1924) [19] with some modification made as per requirement of this investigation as follows: For feulgen the slides were first immersed in 1M HCl at 60°C for 5 to 6 minutes, followed by placement in 1M HCl at room temperature for 1 minute. Then, they were immersed in Schiff reagent for 30 minutes and then transferred to running tap water for 10 minutes. For PAP staining, the slides were stained according to manufacturer’s protocol [19] using a commercially procurable staining kit RAPIDPAPTM (Biolab Diagnostics, Tarapur, Maharashtra, India).
Scoring: Observation was carried out using OLYMPUS BX41 research microscope. The Mn analysis was done at 400 X magnification as per the criteria given by Tolbert et al., and 1000 cells per subject were examined [20].
The frequency of Mn was evaluated as well as other metanucleated anomalies such as Binucleated Cells (BNC), Karyorrhexis (KR) and Karyolysis (KL) were also taken into account. The following criteria given by Tolbert et al., were considered [20]:
Rounded smooth perimeter suggestive of a membrane;
Less than a third of the diameter of the associated nucleus, but large enough to discern shape and colour;
Staining intensity similar to that of the nucleus;
Texture similar to that of nucleus;
Same focal plane as nucleus; and
Absence of overlap with, or bridge to, the nucleus.
Statistical Analysis
Data were subjected to statistical analysis using SPSS software (version 17). Mean percentage occurrence of micronucleated cells and its comparison between PAP and feulgen staining was done using Kruskal-Wallis test. Multiple comparisons among different groups was done using Bonferroni test.
Results
The mean number of Mn cells when compared with PAP and feulgen stain showed very highly significant difference among all the groups. PAP stained smears showed more numbers of Mn than feulgen stained smears [Table/Fig-2,3]. Other nuclear anomalies such as the binucleated cells showed significant result (p-value=0.0034) between Group 1 and Group 2. But when it was compared against Group 3 it was found to be highly significant [Table/Fig-4,5]. Karyorrhexis and karyolysis expressed statistical significant p-value when Group 1 and Group 2 were compared with Group 3. However, when the study groups were compared with each other they were not statistically significant [Table/Fig-4,5].
Descriptive statistical analysis showing mean micronuclei in different groups using feulgen stain.
| Groups | N | Mean Micronuclei | Standard Deviation | Standard Error |
|---|
| With Lesion | 30 | 12.27 | 2.333 | 0.426 |
| Without Lesion | 30 | 10.23 | 1.455 | 0.266 |
| Control | 30 | 3.87 | 1.961 | 0.358 |
Descriptive statistical analysis showing mean micronuclei in different groups using PAP stain.
| Groups | N | Mean Mn | Standard Deviation | Standard Error |
|---|
| With Lesion | 30 | 23 | 2.212 | 0.404 |
| Without Lesion | 30 | 19.93 | 1.257 | 0.229 |
| Control | 30 | 18.50 | 2.330 | 0.425 |
Descriptive statistical analysis showing mean micronuclei in different groups using PAP stain.
| Mean | Number | With Lesion | Without Lesion | Control |
|---|
| BN | 30 | 6.30 | 4.933 | 1.366 |
| KR | 30 | 7.266 | 7.200 | 1.700 |
| KL | 30 | 20.466 | 19.200 | 15.433 |
Statistical analysis showing multiple comparisons using Bonferroni’s test.
| Dependent Variable | Group | Group | p-value |
|---|
| Mn FEULGEN | With Lesion | Without Lesion | 0.0003 |
| With Lesion | Control | <0.0001 |
| Without Lesion | Control | <0.0001 |
| Mn PAP | With Lesion | Without Lesion | <0.0001 |
| With Lesion | Control | <0.0001 |
| Without Lesion | Control | 0.0196 |
| BNC | With Lesion | Without Lesion | 0.0034 |
| With Lesion | Control | <0.0001 |
| Without Lesion | Control | <0.0001 |
| KR | With Lesion | Without Lesion | 0.3012 |
| With Lesion | Control | <0.0001 |
| Without Lesion | Control | <0.0001 |
| KL | With Lesion | Without Lesion | 0.2815 |
| With Lesion | Control | <0.0001 |
| Without Lesion | Control | <0.0001 |
Discussion
Highest frequency of oral cancer in South East Asia is considered to be connected with tobacco use [21]. The mutagenic agents present in tobacco, synthetic and natural chemicals of occupational, environmental, medical and dietary origin causes majority of oral malignancy in humans [22–24]. Structural modification in the DNA of target cells leads to genomic instability in the form of chromosomal abnormalities. Hence, an early diagnostic test would be beneficial to check the progress of premalignant lesion to malignancy [25]. Literature reveals numerous researches on Mn using diverse stains. Most commonly used DNA specific stains are feulgen and around 30% of the studies were done using non-specific DNA stains such as Gimesa, May-Grunwald’s Giemsa and PAP. However, as of now barely any curiosity has been expressed to the effect of divergent staining technique on the outcome of Mn assays [26].
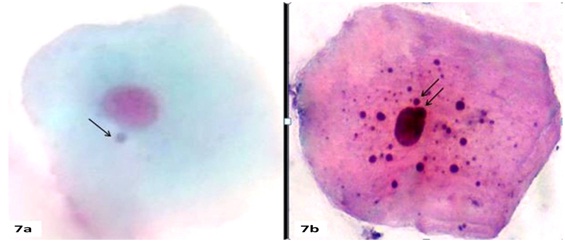
In the present study, we observed a very high number of Mn cells in smears stained with non-specific DNA stain (PAP) [Table/Fig-6,7a] in individuals having tobacco habit with lesion, without lesion and control group in comparison with smear stained with DNA specific stain feulgen [Table/Fig-8a] which was almost twice as high [Table/Fig-6]. These findings are consistent with the recent study of Palaskar Sangeeta et al., [26].
Showing mean percentage occurrence of micronucleated cells and its comparison between PAP and feulgen staining using Kruskal-Wallis test.
| Groups | StainingMean % Mn(±SD) | Comparison | Remark |
|---|
| With Lesion | 23(±2.2) 12.27(±2.3) | p<0.0001 | Very Highly Significant |
| Without Lesion | 19(±1.25) 10.23(±1.45) | p<0.0001 | Very Highly Significant |
| Control | 18.5(±2.3) 3.87(±1.9) | p<0.0001 | Very Highly Significant |
(a) Showing micronucleated cells in PAP stain (100X).
(b) Showing cytoplasmic keratin bodies resembling micronuclei in PAP stain (100X).
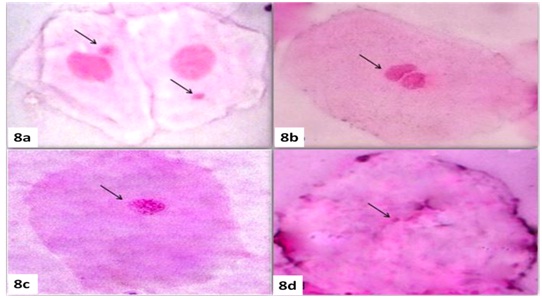
(a) Showing micronucleated cell in feulgen stain (100X).
(b) Showing binucleated cell in feulgen stain (100X).
(c) Showing karyorhexis cell in feulgen stain (100X).
(d) Showing karyolysis cell in feulgen stain (100X).
Mean percentage of occurrence of micronucleated cells and its comparison between PAP and feulgen was very highly significant (p<0.0001). An increased Mn rate in buccal exfoliative cytologies of tobacco, alcohol users and potentially malignant disorders was proven by several authors [9,26–28].
Armen Nerseyan et al., reported almost identical outcome in a study carried out on heavy smokers and non-smokers [12]. They manifested pronounced rise in mean Mn cells in heavy smokers with non-specific DNA stain as compared with DNA specific stain and also observed two times the number of Mn in non specific stain than the specific stain.
Nersesyan A et al., investigated the impact of tar and nicotine content of cigarette on chromosomal damage and observed that there were increased Mn and metanucleated cells on rate of daily exposure and type of cigarette consumed [29]. Stitch investigated the prevalence of Mn initially in India from smokers and betel chewers using Mn assay [23].
Our observation of higher Mn indicates the increase risk of development of malignant lesions, among tobacco users. The occurrence of Mn in anepuloid oral cells is suggestive of chromosomal loss or fragmentation occurring during early nuclear division. It is ascertained from diverse studies that individuals exposed to cytotoxic chemicals are more vulnerable for increase number of Mn along with other nuclear abnormalities [30–32]. Here we evaluated comparatively the frequency of different types of metanucleated cells. Three types of anomalies were counted namely binucleated, karyorrhectic and karyolitic cells.
Binucleated cells [Table/Fig-8b] are paired nuclei that are exceptionally adjacent to each other with identical shape and structure which is speculated to be a cytokinesis check point for aneuploid cells. They are indicative of failed cytokinesis after the final nuclear separation [33]. Hence, the frequency of Binucleated Cells (BNC) in Group 1 and 2 was highly significant when it was compared against Group 3 because it is believed that the carcinogenic agent from tobacco prevent the successful cytokinesis of cells. The frequency of cells showing karyorrhexis [Table/Fig-8c] was compared among all the three groups. Both the study groups showed statistically significant differences when compared with control group. These cells showed disintegration of the nucleus and may be suggesting of late stage of apoptosis.
On examination, the frequency of karyolysis [Table/Fig-8d], we observed that among all the three anomalies karyolytic cells were highly increased compared with control group where as the pattern of induction of other nuclear anomalies varied. This finding was in accordance with several other authors [29,31,34,35].
So in the present study karyolitic cells were higher in comparison with other nuclear anomalies. Increased number of karyolitic cells has significance as these appear in the pre-keratinization process, which depict an adaptive event to cellular trauma because of the chronic effects of masticatory process in the oral mucosa. This anomaly is also evident in necrotic cells and is related to cytotoxicity. Karyorrhexis and binucleated cells formation should be considered as precious morphological stages of karyolysis and Mn in squamous epithelium. Metanucleated anomalies were examined jointly for nuclear degeneration followed by carcinogenesis.
During this process series of sequential events lead to the formation of initially bi-nucleated cells followed by Mn and ultimately KR and KL cells, supporting homeostasis of oral epithelium [11].
However, similar events are also evident in necrosed cells and cannot be considered as reliable marker for augmented DNA damage and cancer risk. It has been stressed by Tolbert el al., that these anomalies are sometimes difficult to infer and may be miscategorised as Mn [20].
Since many studies have shown conflicting results about the reliability of the DNA specific and non-specific stains, we tried to check the reliability of DNA specific feulgen stain over the routinely used non-specific DNA stain PAP. We observed higher numbers of Mn cells in PAP stained smears [Tables/Fig-6] which was statistically very highly significant in comparison with DNA specific feulgen stain in both study groups and control group like many other investigators [12,25,26,36,37]. Nerseseyan et al., revealed raised Mn frequency in Non-specific DNA stains in contrast to specific DNA stains which were in agreement with our results [12]. Results of Grover et al., were also in accordance with our results, they showed increase Mn frequency in PAP and H&E stains compared to feulgen [38]. [Table/Fig-9] summarize different studies that have been done up till now to compare the DNA specific and non-specific stains.
Summary of different studies done to compare the effect of DNA specific and non specific DNA stains.
| Year | Subject studied | Objective | Stains used | Result | References |
|---|
| 1990 | Rat hepatocytesCystadenfibromaCystadenocarcinoma | To compare optical density of nuclei stained by both the stains. | - PAP- Feulgen | PAP stained cells showed substantially higher coefficient of variation value than Feulgen. | A.M. Gurley et al., [37] |
| 1996 | Breast carcinoma (n=40) | To check any interference between PAP and feulgen stain in terms of optical density, DNA indices and histogram profiles. | - PAP- Feulgen | Feulgen revealed low coefficient of varation than PAP. | Angelo Sidoni et al., [38] |
| 2006 | Heavy smokers(n=20)Non smokers(n=10) | Effect of different stains on micronuclei study. | - May –Grunwal – Giemsa and Giemsa.-Feulgen,Acridin orange and DAPI | With giemsa based stains, the frequencies of micronuclei were 4 to 5 folds higher | Armen Nersesyan et al., [12] |
| 2016 | OSMF (n=15)Lichen Planus (n=15)Leukoplakia (n=15)Control (n=15) | To compare the micronuclei frequency using three different stains | - Feulgen-Papanicolaou and Hemotoxylin and eosin stain | Non specific DNA stains showed more number of micronuclei compared to Specific DNA stains. | S Grover et al., [21] |
This high frequency of observation of Mn may not be true Mn as these are actually keratin granules that are found in degenerated cells with nuclear defects. These round cytoplasmic structures does not contain DNA and it might mimic as Mn when stained with non specific DNA stains [Table/Fig-7b].
This apparently increased frequency of Mn observed in PAP stained smears in our study as well as other studies, which have used other non-specific stains like MGG and Giemsa may indicate false positive appearances [12,38,39]. Our investigation specifies that PAP stained nuclei are not dependable and should be used with caution, only for metanucleated analysis. Whereas, feulgen stain is technique sensitive and time consuming but it yielded low values with positive result.
Hence, it can be considered that Mn test is a simple, practical, inexpensive and non-invasive screening technique for clinical prevention and management of subjects under carcinogenic risks, after exposure to genotoxic agents or situations such as abusive and chronic consumption of alcohol, tobacco and or other mutagenic drugs.
Limitation
Limitations that could influence the scoring of Mn in the present study were sampling time since the tissue homeostasis require few days to few weeks. During this turnover time period Mn cells produced in the basal cells could lyse and go missing before making it to the top layers. Therefore, the frequency of Mn may be wrongly estimated in DNA specific stain too.
Conclusion
Increased frequency of MN in tobacco users affirms that tobacco in any form have genotoxic and cytotoxic potential. Metanucleated cells could be the result of many phenomena such as necrosis, apoptosis or may be the adaptive response to cell damage. Hence, we believe that it cannot be regarded as reliable markers for cancer risk. High number of Mn in PAP stained smears indicates that other nuclear anomalies or keratin bodies may be misinterpreted as Mn.
So, PAP stain can be used to identify abnormal cytological changes but not to score Mn. Hence, we state the DNA specific feulgen stain can be used as a reliable stain to interpret Mn over the non- specific DNA stain.