Hemodialysis (HD) patients with end-stage renal disease have compromised immune system and infections are significant contributors to their morbidity and mortality [1]. HD patients are more susceptible to Urinary Tract Infection (UTI) and UTI is the second cause for hospital admission in patients with chronic kidney disease [2,3]. Other common sources of infection in HD patients are cellulitis and bacteremia [4]. Indwelling dialysis catheters (both for HD and peritoneal dialysis) are another source of sepsis associated with early complications [4,5].
When undiagnosed and untreated, UTI is associated with significant complications, which can be fatal. Delayed recognition and over-diagnosis of UTI are relevant issues in the management of symptomatic HD patients. On one hand, UTI may be overlooked in HD patients due to reduced urination and absence of symptoms, thus posing challenges in the clinical diagnosis of UTI [6]. When diagnosed early, UTI can be easily treated and future complications can be avoided [5]. On the other hand, previous studies on asymptomatic HD patients support that pyuria is not always related to infection [2]. However, there are no studies evaluating the diagnostic value of urinalysis parameters (e.g., pyuria and/or bacteriuria, LE, presence of nitrite) in HD patients with fever and/or UTI symptoms. Hence the aim of the present study was to assess the sensitivity, specificity, positive predictive value and negative predictive value of pyuria, bacteriuria, LE and nitrite positivity in symptomatic, febrile and/or septic HD patients requiring hospitalization.
Materials and Methods
Study Population: All HD patients who required acute inpatient care for an admission diagnosis of fever, sepsis or UTI between September 2008 and August 2015 were retrospectively identified.
Fever was defined as a body temperature of 100.4°F or 38°C. The detection of bacteria at a colony count threshold of ≥ 105 bacteria/mL in a urine culture was used as the diagnostic standard for UTI. A clean catch midstream specimen was obtained for urinalysis. Of the total population, two patients with chronic indwelling bladder catheters were identified and another 49 patients underwent bladder catheterization on admission. In these patients urine was obtained using the port in the drainage system after placement (N=49) or replacement (N=2) of the catheter prior to collecting the urine sample. Sepsis was defined as the presence (probable or documented) of infection together with systemic manifestations of infection, according to the Infectious Diseases Society of America (IDSA) guidelines [7].
Study included adult HD patients (older than 18 years), on HD for more than a month, and their urinary output between two dialysis sessions was more than 30 mL [8]. Patients with a suprapubic catheter and diagnosis of interstitial nephritis were excluded. Also, patients for whom urinalysis and urine cultures were drawn after antibiotic initiation or 24 hours after admission were excluded from the study. The study was approved by the Institutional Review Board of New York University Lutheran Medical Center, Brooklyn, New York City, USA.
Statistical Analysis
Clinical, laboratory and demographic characteristics of patients were recorded, including age, gender, cause of end-stage renal disease, presence of fever, sepsis, urinary catheter, urine culture result and their associations with urinalysis parameters were assessed with Pearson’s Chi square and Fisher’s exact test. Predictive discrimination for positive urinalysis parameters was assessed by Receiver-Operating Characteristic (ROC) curve analysis based on maximizing sensitivity and specificity. All analyses were performed using SPSS (version 17, Chicago, IL, USA). The level of significance was set at p<0.05.
Results
Clinical parameters and characteristics of the patients are presented in [Table/Fig-1]. Out of 275 patients assessed (141 males, mean age 73±14.9 years), only 58 (21.1%) were found to have a final diagnosis of UTI on discharge. Among the rest identified sources of infection, respiratory (community-acquired, healthcare-associated, aspiration pneumonia, upper respiratory), bacteremia and GI tract (C. difficile colitis, other infectious colitis, gastroenteritis, cholecystitis, spontaneous bacterial peritonitis) were the most common [Table/Fig-1].
Demographics and clinical characteristics.
| Characteristics | N (%) |
|---|
| Age | 275 (100) |
| <65 | 85 (30.9) |
| ≥65 | 190 (69.1 |
| Gender | 275 (100) |
| Male | 141 (51.3) |
| Female | 134 (48.7) |
| Diabetes mellitus | 163 (59.3) |
| Hypertension | 243 (88.4) |
| Glomerulopathy | 5 (1.8) |
| Polycystic kidney disease | 5 (1.8) |
| Other CKD causes | 23 (8.4) |
| Urinary catheter | 51 (42.1) |
| Fever | 51 (18.5) |
| Sepsis | 115 (41.8) |
| Infection source | 275 (100) |
| Respiratory | 57 (20.7) |
| Skin and soft tissue | 23 (8.4) |
| Bone and joint | 5 (1.8) |
| Bacteremia, catheter-related | 32 (11.6) |
| Bacteremia, non catheter-related | 34 (12.4) |
| GI | 39 (14.2) |
| CNS | 1 (0.4) |
| UTI | 58 (21.1) |
| Unknown/unspecified | 16 (5.8) |
| No infection | 10 (3.6) |
When urinalysis parameters were tested for associations with urine culture positivity and extent of growth, 3 out of 4 tested parameters, including pyuria of different cut-offs, bacteriuria and LE positivity were significantly correlated with positive urine culture (p<0.001, p=0.001 and p<0.001, respectively). However, only pyuria was a significant indicator of urine culture growth at ≥105 CFU/mL (p=0.039) [Table/Fig-2].
Associations of urinalysis parameters with urine culture.
| Urinalysisparameters | Urine culture | p-value | CFU/mL | p-value |
|---|
| Negative | Positive | <105 | ≥105 |
|---|
| N (%) | N (%) | N (%) | N (%) |
|---|
| WBC |
| 5-10 | 35 (62.5) | 24 (24) | <0.001 | 15 (34.9) | 6 (13.6) | 0.039 |
| >10-50 | 12 (21.4) | 20 (20) | 10 (23.3) | 9 (20.5) |
| >50 | 9 (16.1) | 56 (56) | 18 (41.9) | 29 (65.9) |
| Bacteriuria |
| No | 24 (42.9) | 17 (17) | 0.001 | 10 (23.3) | 8 (18.2) | 0.605 |
| Yes | 32 (57.1) | 83 (83) | 33 (76.7) | 36 (81.8) |
| LE |
| Negative | 33 (58.9) | 16 (16) | <0.001 | 10 (23.3) | 5 (11.4) | 0.166 |
| Positive | 23 (41.1) | 84 (84) | 33 (76.7) | 39 (88.6) |
| Nitrite |
| Negative | 55 (98.2) | 97 (97) | 1.000 | 43 (100) | 41 (93.2) | 0.241 |
| Positive | 1 (1.8) | 3 (3) | 0 (0) | 3 (6.8) |
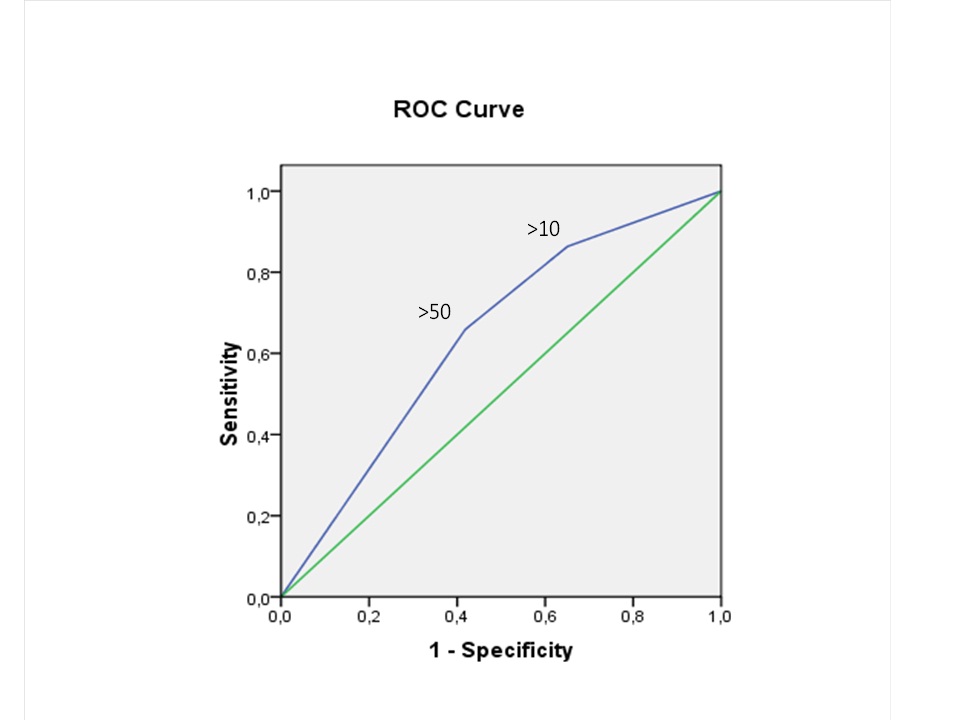
A predictive model for pyuria, was generated at two different cut-offs of >10 and >50 WBC/HPF. The sensitivity and specificity of the WBC/HPF cut-off of 10 for predicting urine culture positivity at ≥105 CFU/mL were 86% and 35%, respectively, whereas, those of the 50 cut-off level were 66% and 58%, respectively [Table/Fig-3]. The corresponding ROC analysis for the two cut-off values of pyuria in terms of a positive urine culture growth of ≥105 CFU/mL disclosed an AUC of 0.64, SE AUC: 0.06 (p=0.024) [Table/Fig-4].
Diagnostic performance of pyuria in identification of positive urine culture.
| Positive urine culture ≥105 CFU/mL |
|---|
| SN | SP | PPV | NPV | LR+ | LR- |
|---|
| Pyuria (WBC/HPF) |
| >10 | 0.86 | 0.35 | 0.58 | 0.71 | 1.33 | 0.39 |
| >50 | 0.66 | 0.58 | 0.62 | 0.62 | 1.57 | 0.59 |
SN = Sensitivity; SP = specificity; PPV = positive predictive value; NPV = negative predictive value; LR+ = positive likelihood ratio; LR– = negative likelihood ratio.
ROC curve for different cut-off values of pyuria and positive urine culture (≥105 CFU/mL). AUC: 0.64. SE AUC: 0.06 (p = 0.024).
None of the urinalysis parameters was associated with a higher risk of fever or sepsis. Thus, the presence of pyuria was not significantly different between afebrile and febrile patients (p=0.266) and same was the case for bacteriuria (p=0.506), LE positivity (p=0.216) and nitrite positivity (p=0.668). Likewise, there was no significant association between presence of sepsis and pyuria (p=0.053), bacteriuria (p=0.599), LE positivity (p=0.137) or nitrite positivity (p=1.000), respectively. However, presence of pyuria and positive LE were more frequently found in patients with urinary catheter (p=0.001 and p=0.031, respectively), but this was not the case with bacteriuria or nitrite positivity (p=1.000 and p=0.434, respectively).
Discussion
In symptomatic patients without kidney disease, pyuria can be a key marker for the diagnosis of UTI. However, in HD patients, the presence of low urine volume and bladder stasis are confounding factors [1].
Hyodo and coworkers compared 75 HD patients with 133 healthy volunteers. The authors reported that pyuria was not a good marker for UTI detection in HD patients [9]. Recently, Vij and coworkers evaluated different cut-off values of pyuria (more than 5, 10, 50, and 100 WBC/HPF) and association with UTI. The specificity of pyuria increased with the increased cut-off value, while sensitivity decreased. They reported that different pyuria cut-off did not seem to have enough sensitivity and specificity as a diagnostic test for UTI. In addition, the presence of nitrites on dipstick had high specificity (94%) but very poor sensitivity (14%-20%) [6]. Mortazavi et al., evaluated urine samples of 90 HD patients and the sensitivity and specificity of pyuria for UTI was 100% and 61.8%, respectively. The positive and negative predictive values were 35.5% and 100%, respectively, leading the authors to suggest that because of the low specificity and positive predictive values, samples with positive pyuria should be cultured to confirm UTI [2].
In our study we further expand this finding of low diagnostic value of urinalysis in symptomatic HD patients with fever and/or clear evidence of sepsis. We observed that pyuria, bacteriuria and LE or nitrite positivity failed to identify patients with significant growth of uropathogens. Particularly for pyuria, neither 10 WBC/HPF cut-off nor the 50 WBC/HPF one, were able to provide an adequately high positive predictive value or specificity. Also both cut-offs had a low post-test probability based on the inappropriately low positive likelihood ratios and the inappropriately high negative likelihood ratios.
Limitation
Limitations of our study include the retrospective assessment of data and difficulty in precise assessment of urinary symptoms in the entire cohort, in most cases secondary to co-morbidities frequently occurring in our elderly patient population, (e.g., incontinence and dementia) which was another limitation. Finally, the evaluation of patients with urinary catheter may also have affected our results, although only patients with clear documentation of aseptic insertion/replacement and sample collection were finally included.
Conclusion
In conclusion, our results suggest that urinalysis is not a reliable diagnostic tool in febrile and/or septic HD patients and a urine culture is needed to further guide treatment, in the appropriate clinical context and after exclusion of other sources of infection, based on thorough history, physical examination and additional culture results. In such patients, physicians should also be vigilant for sources of infection other than the genitourinary tract, which may not be initially evident. Larger retrospective and/or prospective studies are warranted to further clarify the utility of urinalysis in HD patients with infection.
SN = Sensitivity; SP = specificity; PPV = positive predictive value; NPV = negative predictive value; LR+ = positive likelihood ratio; LR– = negative likelihood ratio.