Case Report
Case 1: The patient was a 29-year-old female with gravida 3, para 2, and abortus1 who had a spontaneous full term vaginal delivery. Her prenatal care was normal and intrapartum course was smooth with no use of pitocin. Spontaneous vaginal delivery with normal neonatal outcome occurred after membrane rupture. She developed profuse vaginal bleeding 1 hour and 10 minutes after delivery. The patient was transferred to our hospital one hour and 22 minutes after the bleeding episode. She presented with massive vaginal bleeding and physical examination revealed a soft uterus and uncoagulated blood in the vagina. Her blood pressure was 80/48 mmHg, with a pulse rate of 120/minute, respiratory rate 20/minute, and body temperature 37.7°C. No respiratory distress was noted. Coagulation studies revealed a fibrinogen of 26.1 mg/dL, prothrombin time 32.9 seconds (8–12 seconds), Prothrombin International Normalised Ratio (INR) 3.57, partial thromboplastin time 41.7 seconds (23–35 seconds), Fibrinogen Degradation Product (FDP) 829.1 μg/mL (<5.0 μg/mL), D-Dimer 1162 μg/L (<324 μg/L), Hemoglobin (Hb) 7.6 g/dL, Hematocrit (Hct) 24.0%, and platelet count 101× THSD/μL. She was sent to the operative room for uterine curettage due to suspected retained placenta before the coagulation studies, but no abnormalities were found. Therefore, she received total abdominal hysterectomy because of persistent and uncoagulated bleeding. The vital signs and laboratory data became stable after hysterectomy and transfusion of 14 units of packed RBCs, 12 units of fresh frozen plasma, 17 units of cryoprecipitate, and 12 units of platelets.
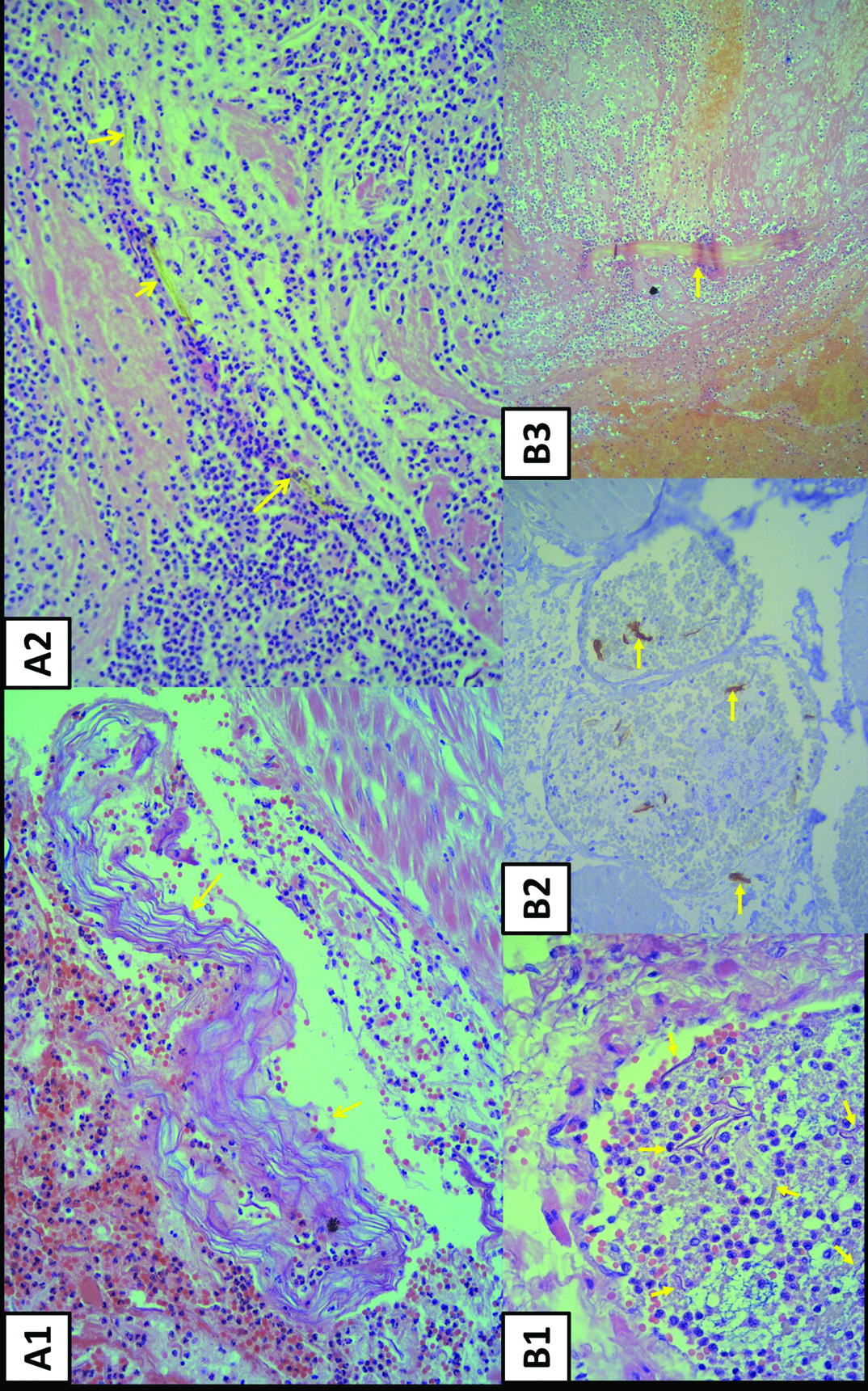
The pathological findings confirmed our diagnosis of AFE and revealed multifocal thrombi with fibrinoid and inflammatory exudates, presence of keratinizing desquamated squamous cells and amorphous materials, and a rare lanugos hair-like structure within the vascular lumen of the cervix and lower uterine segment [Table/Fig-1a1, a2].
(a1) Arrows indicate vascular thrombi with laminated squames (Haematoxylin– eosin stain, original magnification ×200). (a2) Arrows indicate fibrinoid and inflammatory exudates with lanugo hair (Haematoxylin– eosin stain, original magnification ×200). (b1) Arrows indicate presence of intravascular thrombi with squames (Haematoxylin– eosin stain, original magnification ×400). (b2) Arrows indicate the highlighted squames(Immunohistochemical stain for Cytokeratin, original magnification ×200). (b3) Arrow indicates vascular thrombi with lanugo hair(Haematoxylin– eosin stain, original magnification ×100).
Case 2: The patient was a 35-year-old female with gravida 3, para 1, and abortus 1. She was at 39 weeks gestation and was otherwise healthy. She had taken no medication and had no known allergy. She was admitted for delivery. The labour course was uneventful without pitocin augumentation. Thin meconium staining was observed. The patient remained haemodynamically stable throughout the labour and delivered a healthy male baby.
The obstetrician noted steady and profuse noncoagulated uterine bleeding despite perineal laceration repair. Atony uterus was identified. The patient received one dose of methyl ergonovine maleate (0.2mg) intramuscularly and one dose of carboprost tromethamine (0.25mg) intramuscularly along with 1000 μg of misoprostol per rectum. No vaginal clots were observed at any point. Pitocin infusion was instituted at full speed after placental delivery.
A blood sample tested 14 minutes postpartum revealed a Hb of 9.9 g/dL, Hct 29.9%, Platelet 110× THSD/μL, Prothrombine time >150/10 seconds (8–12seconds), PT(INR) >10.0, and partial thromblastin time 39.7seconds (23–35seconds). AFE and associated profound disseminated intravascular coagulation were clinically diagnosed. The patient experienced mild chest pain before insertion of central venous catheter. She was sent for emergent hysterectomy because of uncontrolled bleeding. She exsanguinated despite appropriate medical management and blood components replacement. Total fluid resuscitation, including crystalloid (6500cc), colloid (1000cc), packed RBCs (24 units), whole blood (4 units), fresh frozen plasma (18 units), cryoprecipitate (20 units), and fresh whole blood (19 units), were administered. The pathologic findings from the venous vascular lumens revealed multifocal thrombi, epithelial squames, amorphous materials, and lanugos [Table/Fig-1,b1-b3].
Discussion
AFE is a devastating obstetric syndrome and occurs in 1 in 8000 to 1 in 80,000 pregnancies [1]. AFE occurs during labour in 70%, after vaginal delivery in 11%, and during cesarean section after delivery in 19% of women [2]. Two large population-based cohort studies have reported that the rate of AFE is 14.8 and 6.0 per 100,000 women in multiparous and primigravida deliveries, respectively [3,4]. The actual incidence is unclear because this syndrome is difficult to identify, and its diagnosis remains one of exclusion with possible under reporting of non fatal cases. Because AFE is uncommon, its underlying mechanism remains poorly understood; no single practitioner or institution can have sufficient experience to determine the associated risk factors, clinical course, pathophysiologic factors, or treatment.
Classic presenting symptoms of AFE include respiratory distress, altered mental status, profound hypotension, coagulopathy, and death [1]. According to the study by Clark using the United States National Registry, seizures or seizure-like activity was reported as the initial symptom in 30% of the patients, followed by dyspnea (27%), fetal bradycardia (17%), and hypotension (13%) [2]. More than 50% of the patients reported coagulopathy, which usually accompanied the aforementioned symptoms.
Although there is a general agreement that Disseminated Intravascular Coagulopathy (DIC) almost invariably accompanies amniotic fluid emboli, either clinically or subclinically, it is rarely detected as an initial and isolated symptom. Davies reported an isolated coagulopathy with maternal haemorrhage without cardiopulmonary collapse [5].
Kanayama and Tamura divided AFE syndrome into two groups based on the clinical symptoms: classical type with cardiopulmonary collapse and DIC type with atony bleeding. In patient presents with atonic bleeding, incoagulable vaginal bleeding developed after placental delivery. Uterine atony occurred immediately [6].
The typical laboratory findings were marked decrease in fibrinogen levels and prolong PT PTT levels [7].
This isolated coagulopathy of AFE must be differentiated from the diagnosis of severe abruption placenta, prolonged retention of a dead fetus, septic shock, ruptured uterus, preeclampsia, transfusion reaction, multiorgan failure, and massive trauma or blood loss. DIC associated with AFE typically progresses faster than DIC associated with other causes and symptoms usually develop within 2 hours of delivery. Prompt recognition and treatment of this entity is crucial to survival.
The presence of clotting factors in the amniotic fluid is associated with possible activation of the clotting cascade in the pulmonary vasculature [8–10]. Additional data reported that increased levels of plasminogen activator inhibitor-1 antigen in the amniotic fluid may become active in the maternal circulation, leading to consumptive coagulopathy [8,11]. Recent studies suggest that the pathophysiology might be immune mediated. Non–IgE-mediated anaphylactoid reaction activates the complement cascade and also releases kallikrein and bradykinin, which may explain the another cause coagulation abnormalities [10,12].
In the UK, detection of amniotic components/fetal materials in pulmonary vessels is required for postmortem AFE diagnosis [13]. Although amniotic components can be detected in normal maternal circulation [14]; however, they are rarely detected in maternal lungs during the normal course of pregnancy. In our patients, many squamous cells and even lanugo hairs demonstrated in the uterine vessels. We speculated that the number of squamous cells or lanugo hairs in maternal blood of the patients with AFE may be greater than that in non-AFE patients
Despite inconclusive evidence regarding the cause of the coagulopathy in amniotic fluid emboli, activation of the coagulation system may lead to life-threatening haemorrhage clinically. Treatment of the coagulopathy consists of blood component therapy, including packed RBCs, fresh frozen plasma, platelets, and cryoprecipitate. However, the maternal mortality rate remains extremely high.
Conclusion
Our two patients represent unusual presentations of AFE in which an isolated coagulopathy causes severe postpartum haemorrhage, unlike other patients who present with sudden onset of dyspnea or cyanosis, unexplained shock, and unexplained convulsion, alone or combined with disseminated intravascular coagulation. This coagulopathy following AFE must be differentiated from other causes of postpartum haemorrhage including atonic uterus, placental retention, birth canal laceration, and placenta accreta. However, major haemorrhages in cases described herein were the only clinical manifestations of AFE, which can delay the diagnosis of AFE because an exhaustive search for structural causes of haemorrhage is likely. Therefore, clinicians should suspect AFE when profuse postpartum haemorrhage develops without coagulation and without cardiopulmonary compromise.
Conflict of Interest
The authors have no conflicts of interest.
[1]. Morgan M, Amniotic fluid embolismAnaesthesia 1979 34(1):20-32. [Google Scholar]
[2]. Clark SL, Hankins GD, Dudley DA, Dildy GA, Porter TF, Amniotic fluid embolism: analysis of the national registryAm J Obstet Gynecol 1995 172(4):1158-69. [Google Scholar]
[3]. Kramer MS, Rouleau J, Baskett TF, Joseph K, System MHSGotCPS: Amniotic-fluid embolism and medical induction of labour: a retrospective, population-based cohort studyLancet 2006 368(9545):1444-48. [Google Scholar]
[4]. Gilbert WM, Danielsen B, Amniotic Fluid Embolism: Decreased Mortality in a Population-Based StudyObstet Gynecol 1999 93(6):973-77. [Google Scholar]
[5]. Davies S, Amniotic fluid embolism and isolated disseminated intravascular coagulationCan J Anaesth 1999 46(5):456-59. [Google Scholar]
[6]. Kanayama N, Tamura N, Amniotic fluid embolism: pathophysiology and new strategies for managementJ Obstet Gynaecol Res 2014 40(6):1507-17. [Google Scholar]
[7]. Evans S, Brown B, Mathieson M, Tay S, Survival after an amniotic fluid embolism following the use of sodium bicarbonateBMJ case reports 2014 2014:bcr2014204672 [Google Scholar]
[8]. Estelles A, Gilabert J, Andres C, Espana F, Aznar J, Plasminogen activator inhibitors type 1 and type 2 and plasminogen activators in amniotic fluid during pregnancyThromb Haemost 1990 64(2):281-85. [Google Scholar]
[9]. Hankins GD, Snyder RR, Clark SL, Schwartz L, Patterson WR, Butzin CA, Acute haemodynamic and respiratory effects of amniotic fluid embolism in the pregnant goat modelAm J Obstet Gynecol 1993 168(4):1113-30. [Google Scholar]
[10]. Kobayashi H, Amniotic Fluid Embolism: Anaphylactic Reactions With Idiosyncratic Adverse ResponseObstet Gynecol Surv 2015 70(8):511-17. [Google Scholar]
[11]. Harnett M, Hepner DL, Datta S, Kodali B, Effect of amniotic fluid on coagulation and platelet function in pregnancy: an evaluation using thromboelastographyAnaesthesia 2005 60(11):1068-72. [Google Scholar]
[12]. Busardo FP, Frati P, Zaami S, Fineschi V, Amniotic fluid embolism pathophysiology suggests the new diagnostic armamentarium: beta-tryptase and complement fractions C3-C4 are the indispensable working toolsInt J Mol Sci 2015 16(3):6557-70. [Google Scholar]
[13]. Lee W, Ginsburg KA, Cotton DB, Kaufman RH, Squamous and trophoblastic cells in the maternal pulmonary circulation identified by invasive haemodynamic monitoring during the peripartum periodAmerican journal of obstetrics and gynecology 1986 155(5):999-1001. [Google Scholar]
[14]. Lee KR, Catalano PM, Ortiz-Giroux S, Cytologic diagnosis of amniotic fluid embolism. Report of a case with a unique cytologic feature and emphasis on the difficulty of eliminating squamous contaminationActa cytological 1985 30(2):177-82. [Google Scholar]