Bellini Duct Carcinoma: A Rare Entity
Amit Kumar Mishra1, Ramanitharan Manikandan2, Lalgudi Narayan Dorairajan3, Jayesh Kumar Mittal4, Jinkala Sree Rekha5
1 Senior Resident, Department of Urology, Jawaharlal Institute of Postgraduate Medical Education and Research, Puducherry, India.
2 Associate Professor, Department of Urology, Jawaharlal Institute of Postgraduate Medical Education and Research, Puducherry, India.
3 Professor and Head, Department of Urology, Jawaharlal Institute of Postgraduate Medical Education and Research, Puducherry, India.
4 Senior Resident, Department of Urology, Jawaharlal Institute of Postgraduate Medical Education and Research, Puducherry, India.
5 Assistant Professor, Department of Pathology, Jawaharlal Institute of Postgraduate Medical Education and Research, Puducherry, India.
NAME, ADDRESS, E-MAIL ID OF THE CORRESPONDING AUTHOR: Dr. Ramanitharan Manikandan, Associate Professor, Department of Urology, Jawaharlal Institute of Postgraduate Medical Education and Research, Pondicherry- 605006, India.
E-mail: minks_77@rediffmail.com
Bellini duct carcinoma of kidney derives from collecting duct and is associated with an aggressive course and extremely poor prognosis. Here, we report an interesting case of Collecting Duct Carcinoma (CDC) with Inferior Vena Cava (IVC) thrombus and large retroperitoneal lymph nodes and diffuse desmoplastic reaction. The patient underwent left open radical nephrectomy with IVC thrombectomy and regional lymphadenectomy. Based on morphological and immunohistochemical analysis, diagnosis of collecting duct (Bellini duct) carcinoma was made. Presently, patient is on adjuvant chemotherapy with gemcitabine and cisplatin and under follow-up.
Adjuvant chemotherapy, Lymphadenectomy, Nephrectomy, Thrombectomy
Case Report
A 67-year-old male, presented with a history of gradual weight loss and decreased appetite for six months duration. The patient had a recent onset left flank pain. Clinical examination and renal functions were within normal limits. Contrast Enhanced Computed Tomography (CECT) of the abdomen revealed a heterogeneous enhancing mass of size 5×4×6cm in the mid part of the left kidney along with an infra-diaphragmatic Inferior Vena Cava (IVC) thrombus and multiple regional lymphadenopathy, the largest measuring 4×4cm. A clinical diagnosis of Renal Cell Carcinoma (RCC) was made [Table/Fig-1].
MRI of the abdomen and pelvis showing left renal mass, renal vein and IVC thrombus.
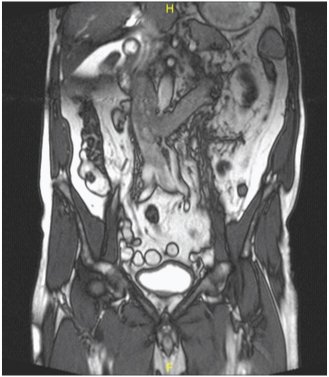
The patient was subjected to left open radical nephrectomy. Intraoperatively, multiple enlarged lymph nodes were found in the perihilar region along with left renal vein and IVC thrombus (Level II) and extending inferiorly up to common iliac bifurcation and adherent to a part of IVC wall. IVC thrombectomy and regional lymphadenectomy was performed.
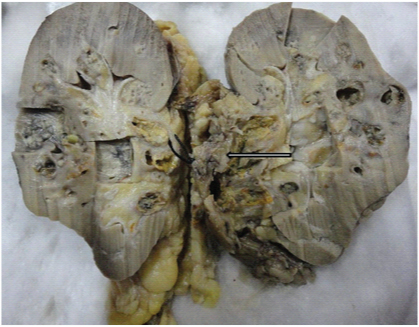
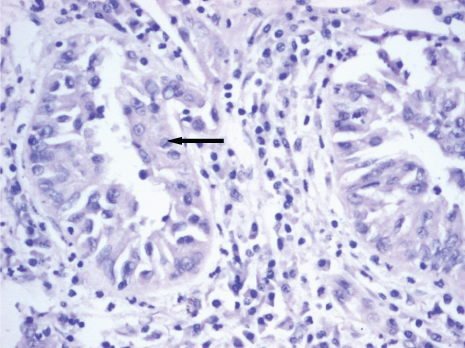
On gross examination, the resected kidney showed an ill-defined greyish white nodular tumour of size 7×4×4cm in the hilar region involving pelvi-calyceal system and extending into medulla and renal vein [Table/Fig-2]. Enlarged lymph nodes showed no evidence of metastasis. Resected margin of renal vein, IVC wall and thrombus showed presence of tumour. On microscopic examination, round to polygonal neoplastic cells with prominent nucleoli and abundant eosinophilic cytoplasm arranged in sheets and tubulopapillary pattern with marked desmoplastic reaction was seen along with areas of necrosis and haemorrhage [Table/Fig-3].
Cut open view of the gross specimen showing greyish white tumour tissue extending from medulla to cortex and involving pelvicalyceal system.
Tubulopapillary pattern of cells (arrow) with marked desmoplastic stromal reaction. 10x magnification (H & E).
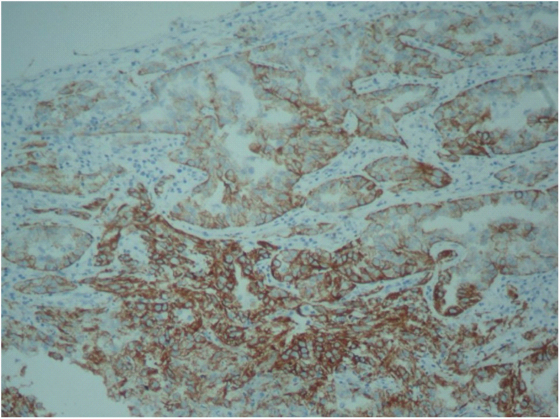
Immuno-histochemical analysis showed that the tumour was positive for high molecular weight cytokeratin (IHC)-CK5/6, Pancytokeratin and Vimentin but negative for CD10, CD117 and thus established the diagnosis [Table/Fig-4]. The final pathologic diagnosis was left renal Collecting Duct Carcinoma (CDC), Fuhrman grade III and the stage was pT3N0M0. Postoperatively, the patient had prolonged paralytic ileus and required insertion of percutaneous drain for a 7×7cm left retroperitoneal collection, which was subsequently removed after two weeks. The patient is presently on adjuvant chemotherapy with Gemcitabine and Cisplatin (GC) and with no evidence of disease relapse on 10 months of follow-up.
Immunohistochemistry showing high molecular weight cytokeratin positivity in tumour cells.
Discussion
CDC is a histological variant of renal malignancy that originates in the duct of bellini of the kidney. CDC has been described by various synonyms like bellini duct carcinoma, medullary renal carcinoma, distal renal tubular carcinoma and distal nephron carcinoma [1,2]. CDC constitutes only about 1% of all RCC. CDC is differentiated from other commonly encountered renal tumours by its characteristic location, radiological features and typical histological appearance [1].
Bellini duct carcinoma is an uncommon variant of RCC with only about 200 cases being reported in literature [2]. In 1986, Fleming and Lewi described it as a medially located, highly aggressive tumour with mixed solid and tubulopapillary patterns and an infiltrating tubular component eliciting a marked desmoplastic reaction. This led to the establishment of bellini duct carcinoma as a distinct type of RCC in literature [3]. The aggressive nature of most of these tumours is evident by the fact that more than 50% of cases presents with metastatic disease [4].
Typical CT findings in CDC include medullary location, heterogeneous and weak enhancement, renal sinus involvement, infiltrative growth, maintained renal contour and a cystic component. But these findings are not specific and histopathology is mandatory to clinch the diagnosis [2].
A desmoplastic reaction around infiltrating tubules, as seen in our case, is unusual in classical RCC. CDC mostly affects younger patients and often presents with nonspecific features such as haematuria, flank pain, a palpable abdominal mass or distant metastasis. Our patient was an elderly male with good performance status, no haematuria, had regional lymphadenopathy and extensive vascular involvement of the IVC and its wall contributing to the unique clinical presentation. To the best of our knowledge, there are only two reported cases of CDC published in Indian literature [5,6].
Radical nephrectomy and regional lymphadenectomy is commonly adopted in the management of CDC. These tumours exhibit highly aggressive behaviour and tendency for early distant metastasis with a median survival of only 12 months even after surgical extirpation [4]. Due to the invariably poor prognosis, Méjean et al., have advocated performing a pre-operative biopsy when radiological findings are highly suggestive of CDC for better decision making [7]. At present, GC regimen is considered as a first-line systemic treatment in metastatic CDC as no other specific chemotherapeutic agent has demonstrated a beneficial effect. GC regimen is advocated as CDC has a mesonephric origin [8].
Treatment with newer oral inhibitors of multiple receptor tyrosine kinases, sunitinib and sorefenib, are all reported to have a favourable effect on the metastatic CDC but their role in therapy is still under evaluation. Procopio and colleagues assessed 13 patients with CDC receiving targeted therapies and 3 patients experienced periods of disease stabilization [9]. Miyake et al., reported partial response of metastatic CDC after sunitinib therapy [10]. Thus the need for prompt detection and multi-modal treatment may be the best strategy to achieve better outcomes in these patients in the current scenario.
Conclusion
At present, the number of accurately diagnosed cases of CDC is limited and follow-up data too miniscule to comment upon the actual survival assessment. The role of targeted therapy in the management of CDC has not been established yet but may be of some benefit based on the limited data available till date. Newer therapeutic modalities need to be explored and developed for treatment and improving the prognostic outcome of the patients with this rare tumour.
[1]. Singh I, Nabi G, Bellini duct carcinoma: review of diagnosis and managementInt Urol Nephrol 2002 1:91-95. [Google Scholar]
[2]. Dason S, Allard C, Sheridan-Jonah A, Management of renal collecting duct carcinoma: a systematic review and the McMaster experienceCurr Oncol 2013 20(3):e223-32. [Google Scholar]
[3]. Fleming S, Lewi HJ, Collecting duct carcinoma of the kidneyHistopathology 1986 10:1131-41. [Google Scholar]
[4]. Chao D, Zisman A, Pantuck AJ, Collecting duct renal cell carcinoma: Clinical study of a rare tumourJ Urol 2002 167:71-4. [Google Scholar]
[5]. Bose D, Das RN, Chatterjee U, Banerjee U, Collecting duct carcinoma: A rare malignancyJ Can Res Ther 2013 9:94-95. [Google Scholar]
[6]. Pavithran K, Hazarika D, Doval DC, Kumar Rajeev, Bellini duct carcinoma- a case reportIJPMO 2006 27(1):38-41. [Google Scholar]
[7]. Mejean A, Roupret M, Larousserie F, Is there a place for radical nephrectomy in the presence of metastaticcollecting duct (Bellini) carcinoma?J Urol 2003 169:1287-90. [Google Scholar]
[8]. Oudard S, Banu E, Vieillefond A, Prospective multi-center phase II study of gemcitabine plus platinum salt for metastatic collecting duct carcinoma: Results of a GETUG (Groupe d’Etudes des Tumeurs Uro-Genitales) studyJ Urol 2007 177:1698-702. [Google Scholar]
[9]. Procopio G, Testa I, Iacovelli R, Treatment of collecting duct carcinoma: Current status and futureperspectivesAnticancer Res 2014 34:1027-30. [Google Scholar]
[10]. Miyake H, Haraguchi T, Takenaka A, Metastatic collecting duct carcinoma of the kidney responded to sunitinibInt J Clin Oncol 2011 16:153-55. [Google Scholar]