Gestational Diabetes Mellitus (GDM), defined as various degrees of glucose intolerance diagnosed or detected for the first time during pregnancy, is the most common metabolic complication of pregnancy [1]. Early diagnosis and adequate treatment are important to prevent complications. Preeclampsia, polyhydramnios, fetalmacrosomia, and operative delivery are some of the complications seen in pregnant women diagnosed with GDM [2].
Reece EA has reported that GDM is a heterogeneous pathophysiological state in which the main mechanism is possibly dysfunction of pancreatic beta cells that manifests itself through increased insulin resistance during pregnancy [3]. Previous studies have shown that GDM has both a direct [4] and an indirect effect [5] on insulin resistance. In most clinical laboratories, the HOMA-IR index is estimated based on the measurement of glucose and insulin in the blood. In most clinical laboratories, HOMA-IR index is estimated with the measurement of glucose and insulin in the blood [6]. It is usually used as a parameter of insulin resistance [6]. Diabetes changes the function and morphology of thrombocytes. MPV is a useful marker that could be used to evaluate platelet morphology. Increased MPV is a direct sign of thrombocyte synthesis and activation. Rao AK noted that a large volume of platelets have more procoagulant activity in diabetic subjects [7]. Elevated Mean Platelet Volume (MPV) is associated with increased platelet aggregation, increased thromboxane A2 and b thrombomodulin release, and increased expression of the receptors of adhesion molecules such as glycoprotein IIb/IIIa and glycoprotein Ib [8]. The aforementioned changes may be associated with an elevated risk of vascular disease [9] and venous thromboembolism. High MPV values cause vein occlusion and a decrease in the concentration of prostacyclin, resulting in vasoconstriction [10–12]. Although previous studies showed an increase in the MPV of pregnant women with GDM compared with a control group [13–16], they did not investigate the impact of high MPV levels on insulin resistance and Apgar scores. In addition, these studies did not attempt to determine the appropriate cut-off MPV levels to predict GDM. On the other hand, the significance and clinical value of Apgar scores and higher glycaemic variability have consistently shown that hyperglycaemia is associated with an increased risk of perinatal morbidity and mortality [17,18].
The aim of this study was to determine the potential value of MPV in predicting poor fetal outcome by examining insulin resistance and neonatal Apgar scores in women with GDM.
Materials and Methods
Study Design
This retrospective study was based on information recorded in the files of all pregnant women referred to our hospital during June 2014–September 2015. The data, which were stored in a computerised perinatal database, consisted of information recorded by the obstetrician directly after the delivery and during the hospital stay. The data were obtained in retrospect after delivery, and trained medical secretaries double-checked all the medical and obstetrical antenatal records, assuring the completeness and accuracy of the database. The obstetrical complications recorded were as follows: pre-term delivery, mild and severe pre-eclampsia, anaemia, amniotic fluid abnormalities, placental abruption and intra-uterine growth restriction, 1 and 5 min Apgar scores and laboratory data. The Ethical Committee and Institutional Review Board of our university faculty of medicine, where the study was conducted, approved the study design. The procedures followed were in accordance with the ethical standards of the responsible committee on human experimentation and with the Helsinki Declaration of 1975 that was revised in 2000.
Inclusion criteria are for control and GDM groups as follows: They all (GDM and control group) had singleton pregnancies and were aged from 20 to 30 years and delivered at the same time as the women in the case group in the same hospital during the study period. The following demographic, clinical and perinatal characteristics were collected: maternal age, parity, smoking, chronic hypertension, and a previous caesarean delivery.
Exclusion criteria’s for control and GDM groups as follows: Subjects with hypertension, chronic inflammatory disease or autoimmune disease, acute or chronic infection, other known malignancy, heart failure, myeloproliferative disorders, hepatic or renal disorders, taking anticoagulation medicine, and Thyroid Stimulating Hormone (TSH) outside normal range were excluded from the study. None of the pregnant women in the GDM group or the control group had pre-eclampsia, eclampsia, pregnancy-induced hypertension or a history of hypertension before pregnancy. None of the individuals had a history of alcohol consumption and a history of smoking or diabetes mellitus. Healthy controls were selected from pregnancy in women who were not diabetic prior to pregnancy with any known diseases. In the healthy control group oral glucose tolerance tests were performed and shown that none of the patients had impaired glucose tolerance or GDM.
One hundred and twenty-six women met the study inclusion criteria. Of these, 18 patients were excluded because of non-compliance with follow-up and a lack of outcome data. The pregnancy was terminated in seven women due to chromosomal abnormalities and/or major fetal malformations, and these women were excluded. We enrolled 101 GDM pregnant women and a control group of 138 healthy pregnant women who gave birth by caesarean section. All the pregnant women included in this study were screened routinely for GDM using the 50g Oral Glucose Challenge Test (OGCT). Women with a 1 h glucose level of 140 mg/dL or more proceeded to a 100 g OGTT as per the guidelines of Carpenter and Coustan [19]. Women with two or more abnormal values were diagnosed with GDM [20]. Only GDM patients whose blood glucose could be controlled through diet and exercise were included in this study.
The initial plan of management requires teaching the patient about diet therapy, exercise recommendations and home glucose monitoring. Normal fasting glucose values during pregnancy are generally in the 60–100 mg/dL range; normal 1-hour postmeal glucose values were below 140 mg/dL. None of the patients in the control group were classified in the medical record coding system as having GDM. The MPV indices were obtained at 24–28 weeks in both groups when the oral glucose challenge test is done and they were tested by the same laboratory in our university. An auto analyser Abbott Cell-Dyn 4000Abbott Park, IL, USA was used to measure the platelet volume in the blood samples [21]. The MPV reference range of our clinic is 7–11.0 fL. Serum glucose was determined by the glucose oxidase method. Serum insulin was measured with an electrochemiluminescence immunoassay on a Roche Diagnostics Elecsys (Roche Diagnostics, Germany). The insulin assay measures concentrations up to 300 mU/mL, with the lowest detection limit of 2mU/mL [22]. The intra-assay Coefficient of Variation (CV) ranged from 7.1 to 8.0 and 5.9 to 7.0 for low and high insulin levels, respectively. Serum glucose was measured with an enzymatic reference method with hexokinase on a Roche Diagnostics Cobas® with an intra-assay CV of 3.2%. The HOMA-IR was calculated for each pair of fasting serum insulin and glucose levels using the mathematical formulas described by Matthews DR et al., [6]. Throughout the pregnancy period, the fetal status was monitored at the same hospital. Obstetricians and neonatologists assessed the pregnancy outcome and the status of the new-born (e.g., term and 1 min and 5 min Apgar scores). Fetal asphyxia was defined as an Apgar score of less than 7 at 5 min postpartum.
Statistical Analysis
Statistical analyses were carried out using the Statistical Package for the Social Sciences (SPSS), version 11.0 (SPSS, Chicago, IL, USA). The Kolmogorov–Smirnov normality test was used to evaluate the distribution pattern of the variables. All parameters were normally distributed and parametric tests were used and data were presented as mean±SD. Demographic characteristics were expressed by descriptive analysis. An independent samples t-test was used to compare continuous variables. Differences between the categorical variables were analysed with the χ2 test. The relationships between different variables were analysed with the Pearson correlation test. To assess the independent determinants of MPV values in the studied population, we used logistic regression analysis. Multivariate linear regression models were used to establish relations between the MPV and Apgar scores at 1 and 5 min with insulin resistance and the correlations between these and other variables. A p-value of <0.05 was considered statistically significant. We obtained the MPV and HOMA-IR in 24-28 weeks when the GDM or normal glucose tolerance has been diagnosed. The ROC analysis was performed to evaluate the ability of the MPV values to diagnose GDM in pregnant women and investigated the correlation of MPVs with insulin resistance and neonatal Apgar scores in the GDM group. Power analysis was conducted; the inclusion of 13 participants per group provided 80% power for detecting differences in plasma MPV levels.
Results
The study population had a relatively young age, slight obesity and a relatively early diagnosis of GDM. In all women with GDM, dietary treatment alone was sufficient to achieve normoglycaemia. [Table/Fig-1] illustrates the clinical characteristics and laboratory test scores of the 101 GDM and 138 normal pregnancies at our university.
Clinical and demographic characteristics of the GDM group and the control group p-value of <0.05 was considered statistically significant.
| GDM Group (n:101) | Control Group (n:138) | p-value |
|---|
| Age (years) | 26.12±5.05 | 26.51±5.14 | 0.78 |
| BMI (kg/m2) | 26.86±2.12 | 26.71±2.06 | 0.86 |
| Glucose (mg/dl) | 89.53±6.59 | 78.50±4.56 | 0.002 |
| First hour glucose (mg/dl) | 178.35±9.38 | | |
| Secound hour glucose (mg/dl) | 156.62±9.51 | | |
| 50 gr OGCT (mg/dl) | 158.91±11.91 | 121.02±8.67 | 0.012 |
| MPV (fl) | 9.16±1.04 | 7.42±0.80 | <0.001 |
| Platelet (n) | 243.8 ± 64.8 | 251.6 ± 45.0 | 0.76 |
| Insulin (mU/l) | 11.11±2.13 | 7.06±1.57 | 0.003 |
| HOMA-IR | 2.45±0.51 | 1.37±0.34 | <0.001 |
| APGAR 1 min | 7.59±1.03 | 8.43±0.70 | <0.001 |
| APGAR 5 min | 8.73±0.82 | 9.68±0.55 | <0.001 |
Maternal age was not significantly different between the groups. The mean maternal age of the controls and patients was 26.51±5.14 years and 26.12± 5.05 years, respectively. The age and BMI of the GDM group and the control group did not differ significantly (p=0.788 and p=0.867, respectively) at 24–28 weeks of pregnancy. The mean Apgar score at 1 min and 5 min was 7.59± 1.03 and 8.73± 0.82, respectively, in the GDM patients. It was 8.43± 0.70 and 9.68± 0.55 at 1 min and 5 min, respectively, in the controls (p<0.001; [Table/Fig-1]). The GDM patients had significantly higher concentrations of fasting glucose and insulin and a significantly higher HOMA-IR than the control group [Table/Fig-1]. Apgar 1 min and Apgar 5 min scores were also significantly lower in the GDM group than the control group [Table/Fig-1].
Among the analysed variables, we found significant, statistically positive relationships correlation in all patients in the GDM group and in the control subjects between MPV values and insulin (r=0.606, p<0.001), fasting blood glucose levels (r=0.535, p<0.001), the 50g oral glucose challenge test 1 h results (r=0.617, p<0.001), BMI (r=0.169,p=0.009), and the HOMA-IR (r=0.635, p<0.001). There was a negatively correlation between the MPV and the Apgar 1 min score (r=-0.504, p<0.001) and the Apgar 5 min score(r=-0.549, p<0.001) and a positive correlation with the HOMA-IR (r=0.635, p<0.001) values in all the subjects in both groups. In addition, there was a negatively correlation between the Apgar 5 min score and the MPV(r=-0.549, p<0.001) and the HOMA-IR (r=-0.589, p<0.001) values in all subjects in the GDM group and the control group. In the multivariate logistic regression analysis, the MPV value was most consistently associated with the Apgar 1 min score (β=-0,370, p=0.006) the GDM group and the control group.
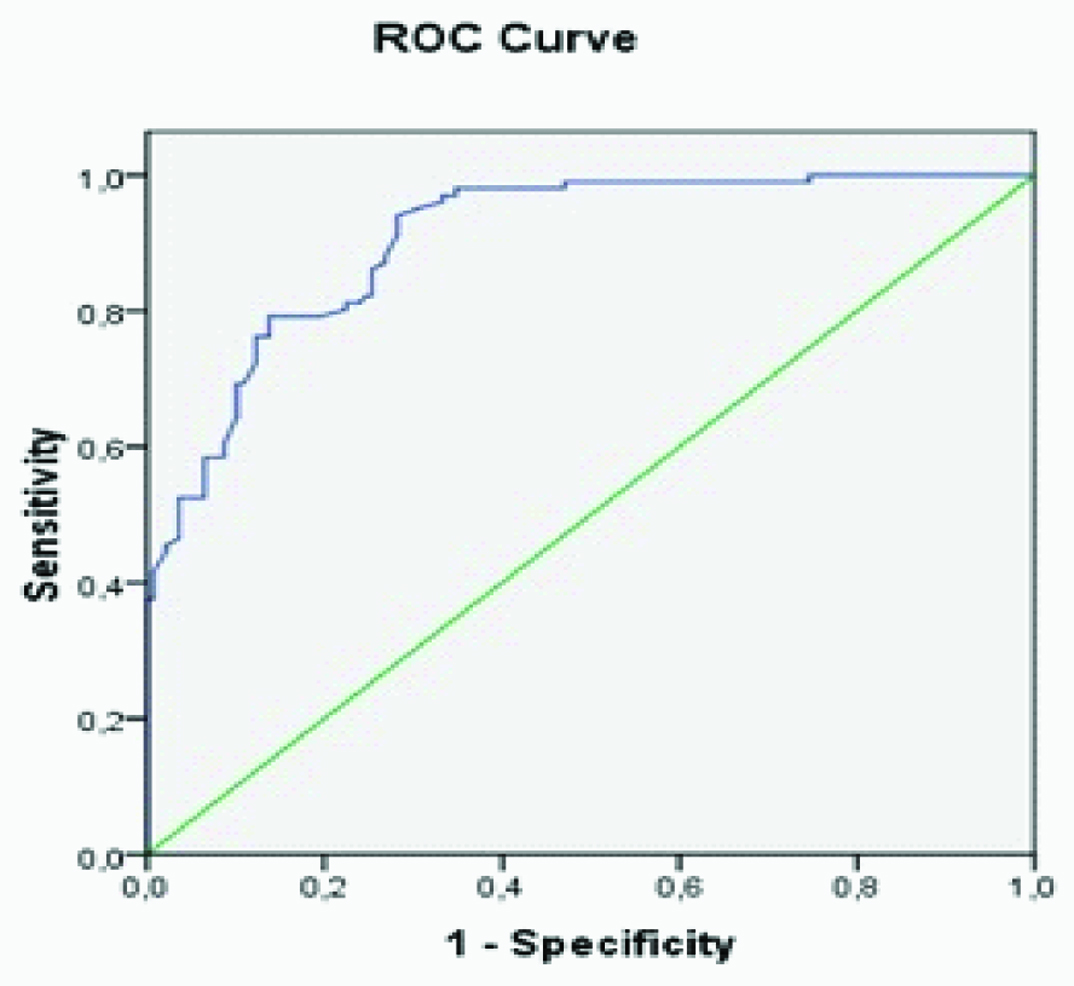
In the GDM group, there was a significant positive correlation between the MPV values and the HOMA-IR, and insulin levels (r=0.227, p=0.02; and r=0.206, p=0.03; respectively). Pearson’s correlation test showed a significant negatively correlation between the MPV values and Apgar 1 min and 5 min scores (r=-0.485, p<0.001; and r=-0.399, p<0.001, respectively). In the multivariate logistic regression analysis, the MPV value was most consistently associated with the Apgar 1 min score (β=-0.387, p=0.003). In the control group, we found a positive correlation between the MPV value and insulin levels, BMI and HOMA-IR values (r=0.193, p=0.02; r=0.329, p<0.001; and r=0.194, p=0.02 respectively). ROC analysis was performed to evaluate the ability of the MPV values at 24–28 weeks to diagnose GDM, as well as could be evaluated the appropriate cut-off value for our population in this study. The area under the ROC curve (AUC) for the MPV to diagnose GDM was 0.906 (p<0.001; 95%confidence interval {CI}, 0.871–0.942). An MPV of >8.0 fL had a sensitivity of 82% and a specificity of 75% for the prediction of GDM [Table/Fig-2].
The area under the ROC curve (AUC) for the MPV to diagnose GDM was 0.906 (p<0.001; 95%confidence interval [CI], 0.871-0942). An MPV of >8.0 fL had a sensitivity of 82% and a specificity of 75% for the prediction of GDM.
Discussion
In this study, we examined the relationship between insulin resistance and Apgar scores with MPV values in women with GDM and healthy controls. The MPV values of the GDM patients were significantly higher than those of the controls. MPV values were inversely correlated with the Apgar score at 1 min and 5 min and positively associated with the HOMA-IR and insulin levels in the patients with GDM. We also found a relationship between the MPV and insulin levels and HOMA-IR values in the control group.
Diabetes, as well as metabolic syndrome and insulin resistance, which is responsible for the syndrome, is a hyper-coagulable condition. Increased platelet reactivity, enlarged activity of the coagulation system and impaired fibrinolysis are typical in diabetic patients [23]. High MPV values may lead to an increase in the production of thromboxane A2, which is specific to thrombocytes. Studies have indicated that changes in the platelet count and MPV reflect the state of thrombogenesis [10–12,24,25]. A study of the placentas of diabetic patients revealed an increased incidence of vascular pathological changes. Research also showed that lesions in obliterative endarteritis, fibromuscular sclerosis and mural thrombosis affected the fetoplacental circulation [26]. As is well known, one of the most significant risk factors in GDM cases is intrauterine foetal loss [27]. This risk factor is due to micro-thrombosis of placental bed vessels and placental infarctions, which result in a conciliation in the fetomaternal circulatory system, leading to low placental perfusion, foetal distress and low Apgar scores [26]. Our data suggest that the greater MPV in GDM patients could be used as a marker for follow-up for diabetic patients. As an elevated MPV may reflect increased platelet activation and a decrease in the concentration of prostacyclin, resulting in vasoconstriction and hypercoagulation or a hyper-coagulable state. Previous research showed that the MPV was significantly higher in GDM populations [13–16]. MPV values can be an effective marker for blood glucose. However, the influence of the MPV on insulin resistance and Apgar scores has not been investigated previously. In our study, in the GDM group and whole group, there was a significant negative correlation between the MPV values and Apgar 1 min and 5 min scores. In the multivariate logistic regression analysis, the MPV value was most consistently associated with the Apgar 1 min score. Starting from this point, we hypothesise that high MPV values may predict low Apgar scores and that they can be used to predict foetal distress. The MPV had a sensitivity of 82% and a specificity of 75%, with a cut-off value of 8fL.
The MPV of patients with type 2 diabetes mellitus showed positive correlations with fasting glucose levels and HbA1c [23] and also in a study of 22 diabetic patients reported high MPV values and significantly reduced MPV values after their blood glucose was reduced [28]. Insulin and insulin resistance have a strong relationship to thrombosis. Insulin resistance increases the functional changes that affect the development of thrombosis in platelets [29]. It is well-established that human platelets have stored insulin receptors in their surface membranes, which induce platelet activation [30]. Several factors are responsible for an increased propensity to thrombosis in patients with insulin resistance: the activation of platelets, vascular dysfunction and decreased vascular endothelial production of prostacyclin and nitric oxide [31–35]. In the present study, we investigated that the direct relationship between insulin resistance and the MPV in patients with GDM. Muscari et al., reported that the percent body fat, blood glucose and ischemic electrocardiographic changes were the main determinants of the MPV in an unselected population of elderly subjects and that the MPV tended to be higher in subjects with a higher HOMA index [36]. In our study, we found that the MPV was correlated with insulin levels and HOMA-IR values in the GDM group. We have hypothesized that the association between insulin resistance and MPV is a likely indicator of platelet activation in patients with GDM. The MPV was also associated with the Apgar scores and insulin resistance in the whole study population. To our best knowledge, there is no studies have been published to examine the relationship of APGAR scores and MPV in the patients with GDM. There is a few data in dealing with APGAR scores and MPV in intrahepatic cholestasis of pregnancy patients. Especially the findings of MPV and indices about low APGAR scores are limited. Recently Oztas Efser et al., suggests that increased MPV levels are associated with preterm delivery and low APGAR in intrahepatic cholestasis of pregnancy patients [37]. They concluded that high MPV could prone the neonate to inflammatory and oxidative lung damage and increased MPV levels were associated with predictive for low APGAR scores. We have evaluated mean platelet volume low APGAR scores in pregnancies with intrahepatic cholestasis of pregnancy [38] vs. normal control pregnancies. In this report we found that MPV values were inversely associated with 5’-Apgar score. Berkowitz et al., reported that the Apgar score and hospital admission showed no relationship with glycaemic control [39]. Likewise, Cousin found no significant relationship between the Apgar score and maternal blood glucose levels [40]. In contrast, Persson et al., reported that the risk of a low Apgar score (<7) at 5 min in diabetic pregnant women increased three times [41]. Weiner has noted that GDM increased the risk of neonatal hypoglycaemia and low Apgar score (<7 at 1 min) in newborns, as compared to the results for women with normal pregnancies [42]. In the present study, Apgar 1 min and Apgar 5 min scores were also significantly lower in the GDM group than the control group.
Limitation
We are aware of the limitations of this study due to its retrospective design. Due to this retrospective design, we do not have data on the impact of glycaemic control after the diagnosis of GDM during pregnancy on the patients’ MPVs. We did not investigate whether altered platelet morphology and function contribute to foetal distress by promoting micro-thrombus formation, decreasing vascular endothelial production of prostacyclin and nitric oxide and reducing small vessel occlusion.
Conclusion
In conclusion, we investigated that the correlation of MPVs with insulin resistance and neonatal Apgar scores in the patients with GDM. Our result seems that a higher MPV value seems to be identifying poor fetal outcome by assessing APGAR score in women with GDM. MPV levels may be a good predictor of fetal outcome and can be used in antenatal monitoring of fetal well-being.
Declaration of interest: The authors report no conflicts of interest. The authors alone are responsible for the content and writing of the paper.