Cigarette smoking is a worldwide problem and one third of the populations are smokers [1,2]. Despite the fact that a decrease in the number of active smokers has been observed, cigarette smoking still plays a significant role among hazardous health-related behaviours. This is particularly disturbing for pregnant women [3]. In addition, it was reported that exposure to the metabolites of cigarette smoke is correlated with cancer in the adult and in utero teratogenicity [4–8]. Soon after inhalation of cigarette smoke, its particulate phase disperses in vapour of air and other gases derived from burning tobacco. The particulate phase of cigarette smoke over all acts as reducing agents, which may play a role in its toxicity [9,10]. The toxicity in the body associated with cigarette smoke and the oxidative stress is caused by release of Reactive Oxygen Species (ROS) [11]. It is also known that cigarette smoking decreases the antioxidant levels in pregnant smokers and their fetuses. Tobacco smoke enhances oxidants by lipid peroxidation, [12,13], protein and thiol oxidation [14,15] and oxidized DNA [16] and antioxidant plays against oxidants in the plasma of pregnant women which results as depleted antioxidant and cause oxidative stress. The oxidative stressed blood which readily reaches to fetus via umbilical cord blood [17]. Smoking or cigarette smoke exposed during pregnancy may stimulate free radical damage in the mother and her growing fetus [18]. Cigarette smoke components adversely affects on reproductive cycle such as folliculogenesis, steroidogenesis, embryo transport, endometrial receptivity, endometrial angiogenesis, uterine blood flow and uterine myometrium which makes the inappropriate environment in prenatal phase [19,20]. Intra-uterine growth retardation and other malformation resulted from hypoxia-ischemia and malnutrition by vasoconstriction reduced by 1/4th of cardiac output [21–23].
Tocopheryl acetate, also known as vitamin-E acetate is strong antioxidant. It is the ester of acetate and tocopherol, which fulfills the depleted level of antioxidants in the mother and to their fetus reduced by maternal cigarette smoke exposure during pregnancy [24–26].
The present study was, therefore, undertaken to evaluate the effect of Tocopheryl acetate on maternal cigarette smoke exposed inbred fetuses of mice.
Materials and Methods
Animal selection
Mice model experiment was done in Department of Anatomy, Institutes of Medical Sciences, Banaras Hindu University, Varanasi, India from January, 2015 to December, 2015.
Male and female swiss albino mice weighing approximately 25 gm (±2g) were taken from the animal house of the Department of Anatomy, Institute of Medical Sciences, Banaras Hindu University. The animals were kept in polypropylene cages, under standard laboratory condition (25° ± 5° C, 12 hr L/D cycle, 55 ± 5 Relative Humidity) with standard animal feed and tap water ad libitum.
The use of animals in this research was approved by and conducted in compliance with the guidelines of Animal Ethics Committee of Banaras Hindu University after submitting the synopsis where number of mice in each group and mode of sacrifice euthanization was mentioned.
Determination of pregnancy
The male and female were mated in the ratio of 2:1. The presence of a vaginal plug in female mice was smeared in a histological slide to observe spermatozoa under microscope 10X magnification. If the spermatozoa were observed, the mice were considered as gestation day 0 (GD 0) and taken their body weight every further subsequent days to assure the pregnancy. Plug positive dams were housed individually in polypropylene cages in the same laboratory conditions.
Experimental design and drug treatment
On GD5 the pregnant mice were randomly assigned to Control group (Group I, n = 6), Tocopheryl acetate group (Group II, n = 6), Soyabean oil group used as vehicle for Tocopheryl acetate (Group III, n = 6) and Cigarette smoke group (Group IV, n = 6), Cigarette smoke plus Tocopheryl group (Group V, n = 6) and cigarette smoke plus soyabean oil group (Group VI, n = 6). From GD 5 to GD 15, the mice in group II and group V along with cigarette smoke exposure were given Tocopheryl acetate at a dose of 200mg/kg/day orally through oral gavage needle. From GD 5 to GD 15, the mice in group III and group VI along cigarette smoke exposure were given Tocopheryl acetate and soya bean oil at a dose of 0.3 ml/mice/day orally through oral gavage 120° angulated blunt needle with a tuberculin syringe. All the group mice were treated with utmost human care.
Smoking system and cigarette smoke exposure
Smoking system apparatus contained three chambers for creation of environment of cigarette smoke exposure to experimental mice, 15x10x10 cm sized air generator contained exhaustion fan one of its surface, which was for production of oxygenated air for ignition of the cigarette. 20x10x10cm sized smoke chamber was meant for ignition and production of smoke, which was connected by an air pipe from the air generator. The produced cigarette smoke was expelled to the inhalation chamber. Cigarette smoke was accumulated in 50x25x20cm sized inhalation chamber which in turn was connected to the smoke chamber. Group IV, Group V along with Tocopheryl acetate oral administration, and Group VI along with soyabean oil oral administration group mice were exposed for 3 times a day for 20 minutes each time which was optimum dose and duration of exposure for the most sustainable cigarette smoke environment in the study.
Fetuses from 50% of dams were collected for external morphology observation and internal observation by histology and rest were left for natural birth for behaviour study. 0n 18th day of gestation, the mother mice was euthanized by using 0.5ml of chloroform soaked a cotton and glass container and kept mice for 30sec for anaesthesia till painless death. The uterus was exposed by a midline excision. The uterus was slit open to observe the resorption and rest of the fetuses along the placenta were collected. Gross features of the collected fetuses were observed along with their placenta. Crown Rump Length (CRL), body weight and the physical examination was done. After dissection, residues of tissues were buried under sterile soil.
Oxidative stress analysis
Fetus were obtained and liver tissues was autopsied, then kept in Phosphate Buffer Solution (PBS) at -20° C and10%(w/v) tissues was homogenated in ice cold PBS (0.1 M, pH 7.4). The homogenate was centrifuged and the resulting supernatant was used for all the biochemical parameters as follows to estimate the oxidative level.
Estimation of Malondialdehyde (MDA) level
MDA was estimated by thiobarbituric acid test protocol from Devasagayam et al., at 530 nm by ELICO- SL-104 double beam UL-UV Spectrophotometer [27].
Estimation of Superoxide Dismutase (SOD) level
SOD was estimated on the inhibition of the formation of NADH-phenazine methosulphate-nitrobluetetrazolium formazon from modified protocol of Kakkar et al., [28]. The colour formed at the end of the reaction and measured at 560nm by ELICO- SL-104 double beam UL-UV Spectrophotometer.
Estimation of Reduced Glutathione (GR) level
GR was estimated on the development of yellow color when 5’5’ dithiobis (2-nitrobenzioc acid) was added to sulphydryl compound. This reaction was read at 420nm by ELICO- SL-104 double beam UL-UV Spectrophotometer.
Statistical Analysis
The experimental results were expressed as Mean + SD. Data was analyzed by ANOVA and Bonfferroni multiple comparison test (BMC tests), using Graph PadPrism (6.0 version). Multiple comparison tests were used for post-hoc analysis in order to compare control and treated groups. The p < 0.05 was considered as significant and p< 0.0001 was considered as highly significant.
Results
Implantation and fetus development: The tabular data shows that highest number of implantation with the maximum dead fetus was found to be in group IV Cigarette Smoke-exposed mice in comparison to other groups [Table/Fig-1]. On administration of Tocopheryl acetate in Cigarette smoke exposed mice increase in number of live fetuses from group V which was near to control live fetuses number in group I [Table/Fig-1]. The maximum number of resorption was observed in Cigarette smoke exposed group IV [Table/Fig-1]. Least pregnancy occurrence was in Cigarette smoke exposed group IV (60%) and group VI (55.55%) [Table/Fig-1,2 and 3]. Administration of Tocopheryl acetate in cigarette smoke increased possibility of pregnancy occurrence in group II (100%) and group V in comparison to control [Table/Fig-1].
Implantation and fetus during uterectomy.
| Group | No. ofAnimals | No. ofImplantation | No. ofDeadFetuses | No. ofLiveFetuses | No. and% ofresorption | No. ofpseudoand %pregnancy |
|---|
| Group I | 6 | 44 | 2 | 42 | 1(2.27) | 1(85.71) |
| Group II | 6 | 43 | 1 | 42 | 0(0.00) | 0(100.00) |
| Group III | 6 | 45 | 6 | 39 | 2(4.44) | 1(75.00) |
| Group IV | 6 | 46 | 10 | 36 | 17(36.96) | 4(60.00) |
| Group V | 6 | 41 | 3 | 38 | 4(9.96) | 2(75.00) |
| Group VI | 6 | 42 | 8 | 34 | 16(3.09) | 5(55.55) |
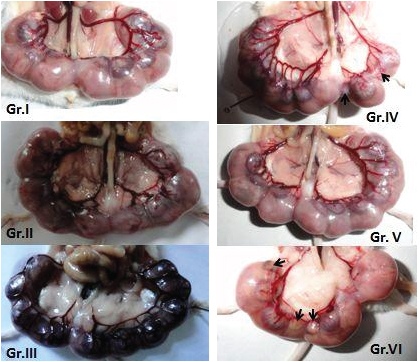
Implantation of Gr. I (control), Gr. II (Tocopheryl acetate treated), Gr.III (soya oil treated), Gr. IV (cigarettes smoke exposed, resorption with placenta in left horn of uterus in black arrows). Gr. V (Tocopheryl acetate induced in cigarette smoke exposed) Gr. VI (soya oil induced in cigarette smoke exposed).
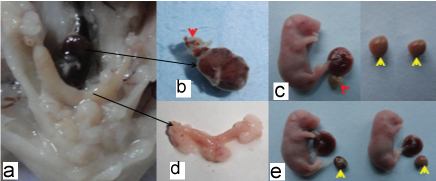
Maternal soya oil induced in cigarette smoke exposed inbred fetus showing; A) ecotpic pregnancy implanted beneath peritonium on lower posterior abdominal wall, enlarged ovaries of both side. B) Dissected placenta with resorption(red arrow) and D) uterus. C) bilobed placenta (red arrow), resoprtions (yellow arrows). E) resorption(yellow arrows) with small placenta along the full term placenta and fetuses.
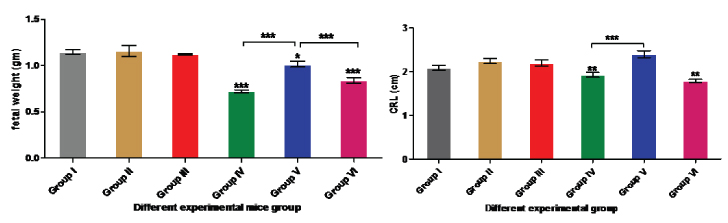
Cigarette smoke exposed pups showed significantly reduced weight (group IV and VI) when compared with control group (p > 0.0001, F = 29.19) followed by group V, [Table/Fig-4,5]. Group II and III showed insignificant increase in their body weight in comparison to control [Table/Fig-4,5]. Group V pups weight showed significant increase in comparison to the group IV and VI [Table/Fig-4,5]. On administration of Tocopheryl acetate, pups weight significantly increased in comparison to group IV and VI [Table/Fig-4,5].
Body weight(gm) of cigarette smoke exposed mice’s inbred strains among different groups. (*p<0.01, ***P<0.0001). Crown rump length (CRL) of Tocopheryl acetate and cigarette smoke exposed mice’s inbred strains among different groups. (**p<0.001, ***P<0.0001) different group.
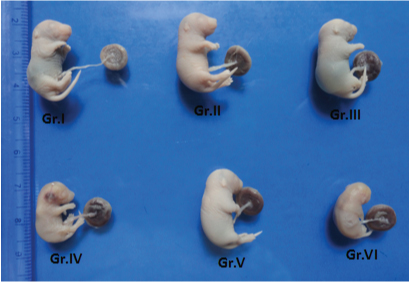
Photograph showing fetuses from different groups: Gr. I (control), Gr. II (Tocopheryl acetate), Gr. III (soya oil), Gr. IV (cigarette smoke exposed), Gr. V (cigarette smoke exposed plus Tocopheryl acetate) Gr. VI (cigarette smoke exposed plus soya oil).
Cigarette smoke exposure showed significant decrease in CRL in group IV and VI whereas the growth was significantly increased in group V in comparison to control (p > 0.0001, F = 10.42), [Table/Fig-4]. Group V showed significant increment in their CRL in comparison to group IV and VI [Table/Fig-4]. On administration of Tocopheryl acetate, pups CRL significantly increased in comparison to group IV and VI [Table/Fig-4].
Gross observation
Gross observation of cigarette smoke exposed maternal fetus had low birth weight phenotype, carpoptosis, lumbar flexion, vertebral mid line haemorrhage, dural venous congestion, jugular venous congestion, thoracic and abdominal visceral haemorrhage, enphalocoele, ectopic pregnancy, discoid placenta, resorption and severe Intrauterine Growth Retardation (IUGR) [Table/Fig-6]. On Tocopheryl acetate administration to cigarette smoke exposed mother, similar malformation were not observed in the fetus.
Maternal cigarette smoke exposed inbred fetus showing; A) carpoptosis (brown arrows), bilobed placenta (red arrow) and normal thickness of umblical cord(black arrow). B) lumbar flexion (blue arrow) and discoid placenta (red arrow). C) Lumbar flexion (blue arrow), dicoid placenta (red arrow). D) severe IUGR fetus showing thinned umbilical cord (black arrow), ill developed cranial end (brown arrow) and lumbar flexion deformities (blue arrow) with full term placenta. E) Severe IUGR fetus showing ill developed ends and extremities, thinned umblical cord with full term placenta. F) & G) showing resorptions. H) Haemorrhage around ear. I) discoid placenta (blue arrow), umblical cord haemorrhage (red arrow). J) Vertebral mid line haemorrhage (red arrow). K) dural venous congestion. L) Umbilical cord haemorrhage (red arrow) milk spot (black arrow), haemorrhage (yellow arrow). M) Jugular venous congestion (blue arrow). N) abdominal visceral haemorrhage (red star), enphalocoele (black arrow). O) Thoracic and abdominal visceral haemorrhage (red star) with enphalocoele (black arrow).
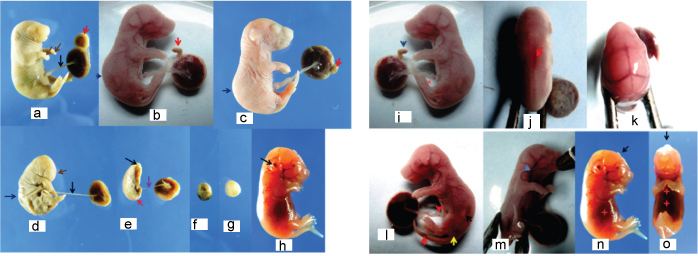
The most frequent occurrence of malformations took in cigarette smoke group (IV) followed by cigarette smoke plus soya oil group (VI), [Table/Fig-7]. Tocopheryl acetate introduced in cigarette smoke exposed group (V) exhibited less occurrence of malformations [Table/Fig-7].
Showing different amomalies such as R (resorption), Intra uterine growth retardation (IUGR), placental anomalies (PA), venous congestion (VA), haemorrhage(H) observed in different groups.
| Group | R | IUGR | P A | VC | H | Other |
|---|
| Group I | 1 | 1 | - | 1 | - | - |
| Group II | - | 1 | 3 | 2 | 3 | - |
| Group III | 2 | 3 | - | 3 | 3 | - |
| Group IV | 17 | 25 | 8 | 15 | 12 | 1 |
| Group V | 4 | 15 | 4 | 4 | 2 | - |
| Group VI | 16 | 27 | 9 | 16 | 11 | 1 |
Biochemical analysis
Malondialdehyde (MDA) activity among different groups showed that Group I, Group II and III were insignificant p>0.05 where as Group IV showed markedly increase in MDA activity in comparison to Group I (p<0.0001) [Table/Fig-8]. Group V had reduced MDA activity in response to administration of Tocopheryl acetate to cigarette smoke exposed mice in comparison to Group IV (p<0.001) [Table/Fig-8].
Oxidative level among fetus in different groups showing oxidative level parameters malondialdehyde (MDA), superoxide dismutase (SOD), reduced glutathione (GR) and catalase (CAT) comparing cigarette smoke exposed group IV with controls and Tocopheryl acetate administrated to CSE comparing with group IV significantly ***p<0.0001, **p<0.0.001 and *p<0.01.
| Subtype | Group I | Group II | Group III | Group IV | Group V | Group VI |
|---|
| MDA(μ mol/mg) | 100.73 ± 34.26 | 100.71 ± 34.20 | 109.91 ± 48.46 | 293.02 ± 81.57*** | 71.43 ± 25.22**** | 338.8 ± 71.33 |
| SOD(mg/ml) | 3.39 ± 1.39 | 3.34 ± 1.54 | 3.23 ± 1.32 | 1.43 ± 0.23*** | 2.45 ± 0.41* | 1.46 ± 0.36 |
| GR(mg/ml) | 0.035 ± 0.003 | 0.035 ± 0.004 | 0.035 ± 0.002 | 0.017 ± 0.002*** | 0.33 ± 0.011*** | 0.018 ± 0.006 |
| CAT(mg/ml) | 0.563 ± 0.009 | 0.554 ± 0.005 | 0.547 ± 0.008 | 0.248 ± 0.005*** | 0.483 ± 0.011*** | 0.261 ± 0.014 |
Superoxide Dismutase (SOD) level among different groups showed that Group I, II and III were within normal limits whereas Group IV had reduced SOD activity in comparison to Group I (p<0.0001) [Table/Fig-8]. Group V had increase SOD in comparison to Group IV (p<0.01) [Table/Fig-8].
Reduced Glutathione (GR) level activity among different groups showed that Group I, II and III were insignificant where as Group IV markedly reduced GR activity in comparison to Group I with significant (p<0.0001) [Table/Fig-8]. Group V had increased GR activity in comparison to Group IV (p<0.0001) [Table/Fig-8].
Catalase (CAT) Level among different groups showed that Group I, II and III (insignificant p>0.05) where as Group IV markedly reduced CAT activities in comparison to Group I with p<0.0001) [Table/Fig-8]. Group V had increased CAT in comparison to Group IV (p<0.0001) [Table/Fig-8].
Discussion
Present study was done to correlate in between teratogenicity and toxicity produced by cigarette smoke and Tocopheryl acetate effects on embryogenic development in their fetus [22,29,30]. In present study, cigarette smoke exposure and Tocopheryl acetate were induced to pregnant mice from GD5 to GD15 which represented embryonic period of human pregnancy. Cigarette smoke used in present study contained the components such as of two phases: the particulate (tar) phase containing stable free radicals and the gas phase, which contains toxins and free radicals are known to cross the placenta reaching levels in the amniotic fluid and fetus that exceed those of the mother [31].
In the present study, the incidence of resorption was high in cigarette smoke exposure (group IV) with high pseudo pregnancy and lowest frequency of pregnancy occurrence which can be a just chance factor, administration of the Tocopheryl acetate after plug formation, there had been reported increased number of pregnancy occurrence. ROS such as the Sulphur Oxide (SO) anion, hydrogen peroxide (H2O2), and the hydroxyl radical formed by water-soluble constituents of tar, damage the fundamental parts of cells and DNA. Even exposure to passive smoke had been linked to decreased pregnancy rates and increased time to conception [31].
Present study showed the Low Birth Phenotype (LBP) in cigarette smoke exposure groups cigarette smoke exposure group IV and VI can be consequence from low food intake, glucose metabolic disorders and altered nutrient supply through blood vessels [32–36]. Present study suggested that significantly low birth weight can be resulted by inhibition of nutrients transfer through placental barrier mid-gestation (GD 12–14) [37]. The average weight of cigarette smoke exposure group IV and group VI fetuses was significantly lower than the other groups which is similar study done by other author exposing (GD 6-18) [38]. Tocopheryl acetate induced group exhibited normal weighing fetuses.
In present study, the average weight and CRL was significantly lowered in Gr. VI (CE) and Gr. VI (CSE + S) in comparison to other groups where Tocopheryl acetate induced group Gr. V mice strains showed significantly ameliorative effects in comparison to other group. CRL morphometry of cigarette smoke exposure fetus was found similar findings that were exposed GD 6-18 [38]. It is possible that smoke exposure-induced decreases in fetal weight are due to delayed embryo implantation and attendant generalized developmental delays. It has been reported that maternal smoking leads to the reduction of fetal weight and CRL which is due to altered placental function and decreased nutrient and oxygen exchange in between mother and fetus [39,40]. The teratogenic mechanism of cigarettes smoke associated with oxidative stress can be multifaceted as it is reported the cytoskeleton, cell motility, zinc methionine, homocystein glutathione metabolism [41]. The principal components believed to be responsible for toxicity are nicotine and benzo (alpha) pyrene through high ROS formation and subsequent OS on the embryo and fetus in addition; a high free radical state can deplete protective antioxidants, namely vitamin-E, beta-carotene, SOD, and catalase [3].
The oxidative stress occurred in cigarette smoke exposure fetus resulted in blood vessel’s wall damage with fatty accumulation which arrests blood flow within areas in tissues which satisfied the obtained in haemorrhagic patches in various place on gross fetus observation in cigarette smoke exposure of present study [42]. Flexion deformities can be explained on the basis of cytoskeletal, cell motility and production of ROS [43]. In the present study, Tocopheryl acetate administration to cigarette smoke exposure, such malformation fetus finding was not observed due to its antioxidant property.
Present study suggested that congenital malformations, low birth weight and other birth defects can be associated with oxidative stress increased in fetus. The oxidative stress was taken in present study MDA as oxidant and SOD, GR and CAT as antioxidants which were significantly imbalanced within fetus. On administration of Tocopheryl acetate, the reduced antioxidants was partially increased the level of antioxidants and decreased the MDA. The reduced oxidative stress level by administration of Tocopheryl acetate also reduced the aforementioned teratogenicity of mice fetus.
Limitation
Efficacy of administration of Tocopheryl acetate in cigarette smoke exposed mice model showed very positive results, these findings may not translate to human pregnancy.
Conclusion
Tocopheryl acetate accelerates the mitochondrial function by balancing effects on oxidative pathway by fulfilling depleted non- enzymatic activities of antioxidants. In the present study, Tocopheryl acetate exogenous oral administration to cigarette smoke exposed pregnant mice, congenital malformation were significantly reduced in comparison to cigarette smoke exposed only group. It was not a complete elimination against effect of cigarette smoke which was potent teratogen which can be one of the among reason that the dose of Tocopheryl acetate and other antioxidant induced might not be sufficient enough during pregnancy period to protect against CSE effects. This study suggests that proper dose of antioxidants and its cofactors dose in cigarette smoke exposed may protect the conceptus against deleterious effect of cigarette smoke exposure. However, Tocopheryl acetate during pregnancy gives healthy pups and reduces congenital anomalies consequence.