Case Report
A 31-year-old man presented with a violaceous nodule over the upper back since one year. It was painless and slow growing. On examination, a firm mobile swelling measuring 1.5x1.5cm was noted. The skin surface showed focal areas of ulcerations. Fine Needle Aspiration (FNA) of this swelling yielded blood. Hence, with a provisional clinical diagnosis of pyogenic granuloma, an excision biopsy was performed. The tissue was sent for histopathological examination. The gross examination revealed a skin covered soft tissue measuring 1.7x1.6cm. The external surface showed ulcerations over the skin. Cut section showed brownish areas.
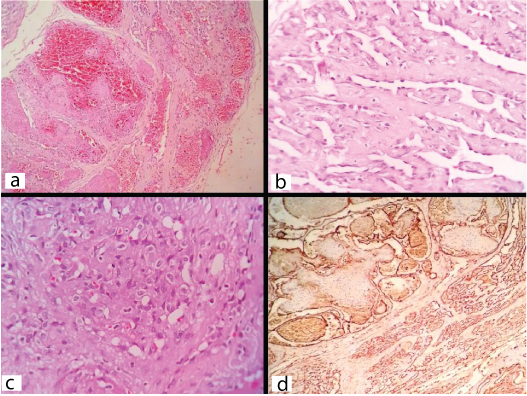
Histopathology of the lesion showed angiomatous areas such as, cavernous vascular channels in the upper dermis lined by benign flattened endothelial cells [Table/Fig-1a]. Foci reminiscent of Retiform Hemangioendothelioma characterized by elongated arborizing vascular channels lined by hobnail endothelial cells embedded in a hyalinized connective tissue stroma were also seen [Table/Fig-1b]. Occasional foci were reminiscent of epithelioid hemangioendothelioma and showed clusters of epithelioid cells with vacuolated cytoplasm. Some of these vacuoles showed intraluminal RBCs [Table/Fig-1c]. There was no atypia or increased mitosis. Small foci of fibrinous necrosis, focal areas of ulceration of overlying epithelium and mixed inflammatory cell infiltrate in the stroma were present. All the surgical margins were free of tumour. Thus, a histopathological diagnosis of composite hemangioma was made. CD34 showed strong positivity in the endothelial cells lining the vascular channels in all these areas [Table/Fig-1d].
Photomicrographs showing: a) Cavernous hemangioma like area (H&E; x100); b) Retiform hemangiendothelioma like areas showing elongated thin vascular channels and hobnail endothelial cells (H&E; x400); c) Epithelioid hemangioendothelioma like areas showing epithelioid cells having round to oval cells and intracytoplasmic lumina showing RBC(H&E;x400); d) Immunohistochemistry showing CD34 positive endothelial cells in various areas (IHC;CD34).
At the end of 5 months of follow up after wide local excision beyond surgical margins the patient was free from metastasis and local recurrence.
Discussion
Composite hemangioendothelioma (CHE) was first described in 2000 by Nayler et al., [1]. It is a vascular tumour of intermediate malignancy and commonly occurs in young to middle aged adults, but can present at any age from birth to 75-year-old. It usually presents on the skin and soft tissue of upper and lower extremities, especially the lower leg and foot [2]. Other sites are the head and neck region chiefly the tongue, mandibular vestibule, cheek mucosa, scalp, nose, and hypopharynx. They can develop in trunk, axilla, upper arm, upper back, thigh, achilles tendon mediastinum, spleen and kidney [3].
The lesions may be erythematous or violaceous. They may be solitary or multiple. They present as poorly circumscribed nodules, plaques, or ulceroproliferative lesions. Multiple sites are affected in some patients. The individual nodules can range from 0.7 to 30 cm in size [2]. The duration of the disease can vary from few months to several years. The female to male ratio is 3:2 [2]. It may occur in association with Maffucci syndrome or Kasabach-Merrit syndrome [2].
Histopathologically CHE is a complex heterogenous vascular tumour with infiltrative margins [2]. It also exhibits inter and intra lesional variability. An admixture of components resembling benign, intermediate, and malignant vascular tumours is seen [2]. Composite hemangioendothelioma contains areas that resemble at least two of the following tumours: spindle cell hemangioma, epithelioid hemangioendothelioma, retiform hemangioendothelioma, Kaposiform hemangioendothelioma, papillary intralymphatic angioendothelioma or angiosarcoma like areas and these areas merge imperceptibly with one another [3,4]. Areas of cavernous hemangioma, lymphangioma, or arteriovenous malformation are rarely seen [4]. Therefore, apart from these lesions, polymorphous hemangioendothelioma, cavernous and capillary hemangiomas, pyogenic granuloma, hobnail hemangioma, and cutaneous angiomatosis are some of the other differential diagnoses that can be considered in this case.
The spindle-cell hemangioma/hemangioendothelioma can develop in any age and usually affects the males. It can be multicentric. Association with Maffuci syndrome has been noted. It displays thin-walled cavernous channels that may contain thrombi or phleboliths, solid areas of bland spindle cells with slit-like vascular spaces, and plump endothelial cells arranged either in groups or lining vascular channels, that may have intracytoplasmic vacuoles [5]. However, presence of retiform hemangioendothelioma like areas and lack of spindled cells lead to the diagnosis of composite hemangioendothelioma in the index case, although the features like cavernous areas with fibrin thrombi and epithelioid hemangioma like areas similar to spindle cell hemangioma were discernible.
Retiform HE usually arise in the extremities of young adults and multiple lesions can be present at times. They exhibit branching blood vessels with thin, anastomosing channels, mimicking the rete testis. These vessels are lined by monomorphous typical prominent endothelial cells with a hyperchromatic and hobnail appearance and are in the sclerotic stroma infiltrated by lymphocytes [5]. These vascular channels may appear dilated or cavernous at the areas near the epidermis [6]. However these lesions do not exhibit epithelioid hemangoendothelioma like areas unlike the present case.
Kaposiform HE shows infiltrative nodules of spindle cells, along with crescentic vascular channels [2]. Our case lacked spindle cells. Epithelioid hemangioendotheliomas usually occurs in adults. Females are involved more frequently than males. Microscopically they show cords and nests of round endothelial cells with abundant eosinophilic cytoplasm and round or indented nuclei and Intracytoplasmic lumina with red blood cells. The stroma is scanty or myxoid and may exhibit mononuclear cell infiltrates. Mitosis, atypia and necrosis are rare [6]. Again, these lesions are also devoid of other patterns like retiform hemangioendotheliomas and cavernous hemangiomas like areas.
Polymorphous hemangioendothelioma has been described in soft tissues and lymph nodes. Histopathologically a combination of undifferentiated solid areas with evident angiomatous pattern and uniform cytologic elements are seen unlike composite hemangioendothelioma. They have uniform cytological features and lack epithelioid spindle cell and angiosarcoma- like areas [2,5].
Dabska tumour or endovascular papillary hemangioendothelioma are usually seen in children and frequently affects the head and neck area. They exhibit dilated vascular lumina showing glomeruloid papillary tufts lined by plump endothelial cells [6]. These lesions also lack other patterns like retiform and epithelioid hamengioendothelioma like areas.
Pyogenic granulomas and capillary hemangiomas show lobules of tiny capillaries lined by plump endothelial cells having benign oval nuclei embedded in a fibroconnective tissue stroma. The stroma shows edema and inflammatory cell infiltrates in addition to capillaries pyogenic granuloma [6]. The present case showed hemangioendothelioma like areas in addition to these areas.
Hobnail hemangioma presents clinically as a single brown to violaceous papule surrounded by a thin pale area and a peripheral ecchymotic ring. Microscopically, in the initial stages, the papillary dermis shows dilated vessels lined by a single layer of prominent epithelioid-like endothelial cells with hobnail appearance and solid intraluminal projections. The deep dermis shows, angulated and slit-like vascular spaces concentrated around sweat glands, frequently forming small hemangiomatous nodules, dissecting the collagen bundles. Extravasation of RBCs, inflammatory lymphocytic aggregates and fibrin thrombi are present. In later stages, the vascular lumen is collapsed and extensive fibrosis and deposition of hemosiderin in the stroma are noted [7]. The characteristic clinical appearance, lack elongated branching delicate vessels of retiform hemangioendothelioma differentiate this from composite hemangioendothelioma.
Cutaneous angiomatosis affects children or young adults and frequently involves large body surface area. Histologically, it shows mixture of fat and large venous, cavernous vascular spaces along with capillary-sized vessels. The veins show incomplete muscular layer and small vessels can be seen in the walls of larger vessels [8]. They lack epithelioid and retiform hemangioendothelioma like areas.
Histopathologically, cutaneous angiosarcoma displays inter-connected irregular neoplastic vascular channels arranged in a sinusoidal fashion and invasion of surrounding tissues. Presence of cytological atypia, numerous mitotic figures, and absence of hobnail endothelial cells differentiate it from retiform hemangioendothelioma [6].
The tumours are positive for CD31 and CD34. Prox-14 and D2-405 may be positive in areas of lymphangiomatous component. Weak immuno-expression for factor VIII-related antigen and von willi brand factor [5] are also noted. Smooth muscle actin (SMA), cytokeratin AE1/3, EMA S-100, desmin, KP1, and human herpes virus 8 [3] are negative in the tumour cells. The stromal cells may show SMA positivity [5]. The angiosarcomatous component shows a greater proliferation index Ki-67 has a range of expression from 0.1% to 14.9% [2–5].
Local recurrence occurs in up to 50% of cases. Regional lymph node or soft tissue metastasis and distant metastasis are rare. Presence of satellite lesions in case of cutaneous lesions may indicate aggressive behaviour. Lumbar spine and pelvic bones, soft tissue, lung and liver are the common sites of hematogenous metastasis [2].
Surgical excision beyond the clinical margins is the treatment of choice. Amputation of affected extremity gives better results [2]. Other modalities include interferon [1,4] and electron beam [2]. The role of adjuvant chemo or radiation therapy is yet to be evaluated due to paucity of cases [3].
Evaluation of the regional lymph nodes using ultrasonography, CT, or magnetic resonance imaging preoperatively and at periodic intervals may be useful in preventing metastasis and local recurrences [3].
Conclusion
We presented a rare case of composite hemangioendothelioma. The knowledge of existence of this tumour and its differential diagnosis helps us to make appropriate diagnosis. It is a tumour of intermediate malignant potential; hence, ruling out its benign and malignant mimics will contribute to better patient management.
[1]. Nayler SJ, Rubin BP, Calonje E, Chan JK, Fletcher CD, Composite hemangioendothelioma: a complex, low-grade vascular lesion mimicking angiosarcomaAm J Surg Pathol 2000 24:352-61. [Google Scholar]
[2]. Zhang J, Wu B, Zhou GQ, Zhang RS, Wei X, Yu B, Composite hemangioendothelioma arising from the kidney: case report with review of the literatureInt J Clin Exp Pathol 2013 6(9):1935-41. [Google Scholar]
[3]. Mahmoudizad R, Samrao A, Bentow JJ, Peng SK, Bhatia N, Composite hemangioendothelioma an unusual presentation of a rare vascular tumourAm J Clin Pathol 2014 141:732-36. [Google Scholar]
[4]. Chu YC, Choi SJ, Park IS, Kim L, Han JY, Kim JM, Composite hemangioendothelioma- a case reportThe Korean Journal of Pathology 2006 40:142-47. [Google Scholar]
[5]. Requena L, Kutzner H, HemangioendotheliomaSeminars in Diagnostic Pathology 2013 30:29-44. [Google Scholar]
[6]. Weiss SW, Goldblum JR, Enzinger and Weiss’s Soft tissue tumours 2008 5th edChinaMosby Elsevier [Google Scholar]
[7]. Kakizaki P, Valente NYS, Paiva DLM, Dantas FLT, Gonçalves SVCB, Targetoid hemosiderotic hemangioma - Case reportAnais Brasileiros de Dermatologia 2014 89(6):956-59. [Google Scholar]
[8]. Stratton JS, Billings SD, Vascular tumours of intermediate malignancy: a review and updateDermatol Sinica 2009 27:140-53. [Google Scholar]