Radiation Therapy in Paediatric Orbital Granulocytic Sarcomas: Experience from a Tertiary Cancer Center
Sushmita Pathy1, Bhanu Prasad Venkatesulu2, Supriya Mallick3, Subhash Chander4
1 Additional Professor, Department of Radiation Oncology, All India Institute of Medical Sciences, New Delhi, India.
2 Senior Resident, Department of Radiation Oncology, All India Institute of Medical Sciences, New Delhi, India.
3 Senior Research Associate, Department of Radiation Oncology, All India Institute of Medical Sciences, New Delhi, India.
4 Professor, Department of Radiation Oncology, All India Institute of Medical Sciences, New Delh, India.
NAME, ADDRESS, E-MAIL ID OF THE CORRESPONDING AUTHOR: Dr. Sushmita Pathy, Department of Radiation Oncology, Second floor, All India Institute of Medical Sciences, Ansari Nagar, New Delhi-110029, India.
E-mail: drspathy@gmail.com
Introduction
Orbital Granulocytic Sarcoma (OGS) is an uncommon manifestation associated with haematological malignancies. Chemotherapy remains the cornerstone of the treatment. The role of radiation is not well-defined.
Aim
To evaluate the effect of radiation in OGS and to define an optimal dose for achieving adequate local control.
Materials and Methods
This was a retrospective analysis of 11 patients who received radiation therapy to orbit for Granulocytic Sarcoma (GS) between 2007 and 2014 at a tertiary cancer center in India. Radiotherapy was planned by three dimensional conformal (3DCRT) techniques. Demographic and disease characteristics, including clinical, imaging, histopathology and treatment details in this patient cohort were recorded and their response to therapy was assessed.
Results
The median age was 7 years (Range: 2-16 years). There were 3 female and 8 male patients. Eight patients were diagnosed as Acute Myelogenous Leukemia (AML), two patients had Primary Orbital Granulocytic Sarcoma (POGS) and one had bi-phenotypic leukemia. Median dose was 24.5Gy (Range-15-45 Gy). Two anterior oblique field design were used most commonly. Out of 11 patients, 5 (45.4%) had complete response, 3 (27.27%) had partial response, 1 patient had stable disease (9%) and 2 developed progressive disease (18%). Median follow-up was 24 months (Range 24-84 months). At last follow-up, 7 (63.6%) patients were alive and 4 patients (37.4%) were dead due to progressive disease.
Conclusion
In patients with residual orbital disease after chemotherapy, low dose radiation can be used to improve local disease control and improve quality of life. Local conformal radiotherapy of 24-30 Gy in conventional fractionation appears optimal with excellent local control and minimal morbidity.
Conformal, Local control, Orbit, Radiotherapy
Introduction
Granulocytic Sarcoma (GS) is an uncommon solid tumour comprising of cells of myeloid precursor. It may occur as a separate entity as primary GS or secondarily associated with Acute Myelogenous Leukemia (AML), Chronic Myeloid Leukemia (CML), Myelo-Dysplastic Syndrome (MDS), Polycythemia Vera (PV), myelofibrosis and essential thrombocytosis [1]. It is commonly associated with Acute Myeloid Leukemia (AML) and hence known as extra medullary manifestation of AML. Incidence of GS ranges from 0.7-9% in patients with AML, CML, MDS and Polycythemia Vera (PV). Primary GS are known precursors of occurrence of haematological malignancies. The most common sites of GS include bone, skin, orbit, lymph nodes, mediastinum, small intestine, paranasal sinuses, epidural sites, lung, uterus and ovaries [2,3]. Chemotherapy is the cornerstone of therapy. However, many patients present with persistent orbital lesion; often unresponsive to systemic therapy. Leukaemic cells are known to be extremely radiosensitive. We hypothesized that this radio sensitivity could be exploited in Orbital Granulocytic Sarcoma (OGS) to obtain relief from compressive symptoms and to reduce visual deterioration produced by Leukaemic infiltrates. Also, this may be useful to avoid enucleation and maintain cosmesis. This may provide a window to achieve better outcome. Prospective data evaluating the role of radiation in GS is lacking and only few isolated case reports and small case series report durable local disease control [4]. These encouraging results prompted us to analyse our data and evaluate local control, treatment compliance and patterns of failure in orbital GS patients treated with conformal radiotherapy. We also intended to find the optimal dose for eliciting a response in such patients.
Materials and Methods
This was a retrospective analysis of 11 patients who received radiation therapy to orbit for GS between 2007 and 2014 at a tertiary cancer center in India. [Table/Fig-1,2a-c] shows a clinical picture and a CT scan. Patient’s ≤20 years with confirmed histopathological diagnosis of primary OGS or secondary to haematological malignancies who received radiation were included in the study. Pre-treatment evaluation included detailed history and physical examination, complete blood count, liver and renal function test, computed tomography of orbit. A bone marrow aspiration and biopsy was done in all cases. A biopsy from the orbital mass was done in the absence of a diagnosis of AML. However, the patients treated for AML and subsequently developed GS were treated based on clinico-radiologic characteristics. Demographic and disease characteristics, including clinical, imaging, histopathology, and treatment details in this patient cohort were recorded and their response to therapy was assessed. The study was approved by Institute Ethics Committee Ref. NoIEC/NP-392/9.10.2015.
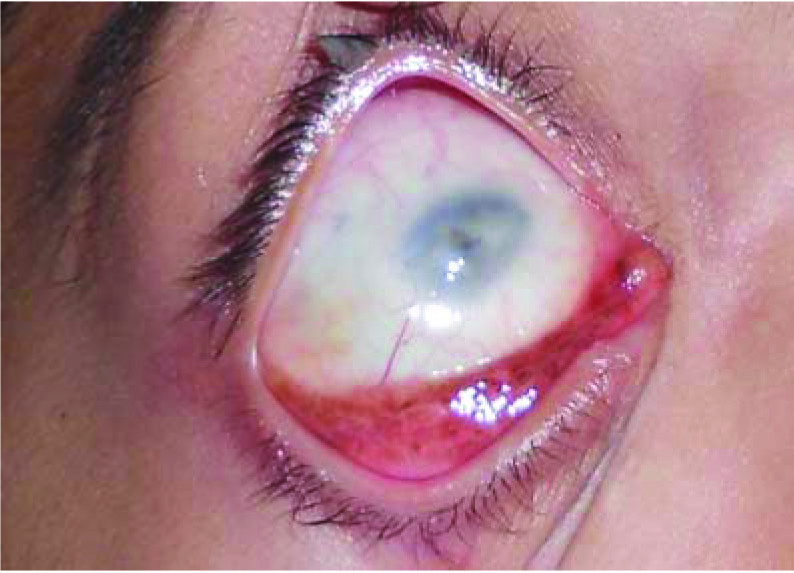
Clinical photograph of a patient with orbital granulocytic sarcoma showing an erythaematous fleshy mass involving right lower palpebral and bulbar conjunctiva with corneal opacity.
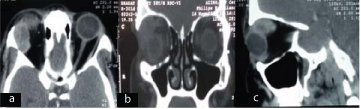
Computed tomography scan of a patient with orbital granulocytic sarcoma showing a 4.5 x 3 x 3 cm well-defined homogenous extraocular mass with intraconal and extraconal component indenting the globe and displacing the optic nerve on contrast enhanced axial (a), coronal (b) sagittal (c), images.
Treatment Details
Chemotherapy: Chemotherapy was the mainstay of treatment and included induction with Daunorubicin (60-90mg/m2 IV day 1 to day 3) and Cytosine Arabinoside (AraC) (100-200 mg/m2 IV day 1 to day 7). Following induction chemotherapy, bone marrow biopsy was repeated and patients in Complete Response (CR) were further planned for consolidation chemotherapy with high dose Ara C 3gm/m2 q12 hour for 3-4 cycles. Patients who developed GS were referred to radiation in case of pain or symptoms that require palliation.
Radiotherapy: The patients referred for radiation were evaluated in a multi-disciplinary clinic in the presence of a medical oncologist, radiation oncologist and ophthalmologist. A planning CT scan (with intravenous contrast) was done with thermoplastic immobilization cast in Philips large bore CT scanner with 3 mm slice thickness extending from the vertex to the C6 vertebra. Radiotherapy consisted of Linac based 3D-CRT (Elekta Medical Systems Crawley UK). The Gross Tumour Volume (GTV) included the enhancing tumour seen on planning CT scan. The whole orbit was included in the Clinical Target Volume (CTV). A uniform expansion of 5 mm was given all around the CTV to generate the Planning Target Volume (PTV). Two anterior oblique fields were commonly used for radiation planning. Contra lateral optic structures including lens, lacrimal gland, and optic nerve, and optic chiasma, brainstem, spinal cord and bilateral temporal lobes and cochlea were delineated as critical organs at risk. At radiotherapy planning and evaluation, high priority was given to achieve a conformal dose distribution covering the PTV followed by maximal sparing of the critical organs at risk. Dose prescription was made at 90% isodose.
Response Assessment
Patients were monitored during the course of radiotherapy and appropriate symptomatic treatment was offered for acute morbidities. Response was assessed using response evaluation criteria in solid tumours (version 1.1) [5].
At our institute we practice regular clinical examination every 3 months and with CT imaging at an interval of 6 months after completion of treatment for the first 2 years and subsequently clinical examination with imaging every 6 months till 5 years or earlier in the presence of clinical suspicion.
Statistical Analysis
Data was analysed and categorical variables were summarized by frequency (%) and quantitative variables were summarized by median and range. Survival outcomes were calculated from the time of diagnosis. Local Progression Free Survival (PFS) was planned to calculate from the time to radiation to local progression. Kaplan Meier method was used for survival analysis. SPSS version 16.0 was used for all statistical analysis.
Results
Patient characteristics are summarized in [Table/Fig-3]. The median age was 7 years (range: 2-16 years). There was a slight male preponderance with male to female ratio of 8:3. Proptosis was the most common symptom followed by earache and bleeding in one patient each. Median duration of symptom was 4 months (range 1-7 months). Primary Orbital Granulocytic Sarcoma (POGS) was diagnosed in 2 (18.18%) patients. Amongst the others, GS was commonly associated with AML-M2 in 6 (54.4%), AML-M4 in 2(18.18%), bi-phenotypic leukemia in 1(9%). CECT of the orbit was done in 7(63.63%), Magnetic Resonance Imaging (MRI) of the orbit in 2(18.18%) and whole body 18F-FDG positron emission tomography with computed tomography (PET-CT) in 1(9%) patient. An extraocular mass in extra conal and intra conal compartment was the most common radiologic finding. Two patients showed involvement of lacrimal gland and another two patients had disease extension in the maxillary sinus and nasal cavity. Ten patients underwent bone marrow evaluation. Eight patients with underlying haematological malignancies had blasts ranging from 8% to 90% in bone marrow at baseline. There was no evidence of blasts in bone marrow or Peripheral Blood Smear (PBS) in 2 patients with primary orbital GS. Cerebrospinal fluid (CSF) analysis was carried out in all patients and only one patient had cytological examination positive for blast cells. Regarding the temporal association of diagnosis of AML and orbital GS, two (18.18%) patients presented as primary orbital GS, five has synchronous presentation of orbital lesion and haematological malignancies. One patient developed orbital GS during the course of chemotherapy for AML and three patients developed orbital GS after the completion of consolidation chemotherapy.
Summarizes patient characteristics.
| Age(years) | Sex | Diseaseassociation | Bone marrowblasts | Post chemo status in orbit | Response toradiation | Status at last follow-up |
|---|
| 2 | M | AML M2 | 35% | Residual disease | SD | Alive at 13.3 months |
| 3 | F | POGS | Negative | POGS | CR | Disease free at 75 months |
| 3 | M | AML M2 | 40% | Developed OGS during leukemia treatment | PD | Alive with disease at 18.3 months |
| 5 | M | Bi-phenotypic leukemia | 90% | Post completion of treatment developed OGS | CR | Disease free at 29.2 months |
| 6 | F | AML M4 | – | Residual disease | PR | Expired after 16.4 months |
| 6 | M | AML M2 | 18% | Residual disease | PR | Expired after 10.9 months |
| 8 | F | AML M2 | 8% | OGS developed post completion of treatment | CR | Alive with disease at 8.5 months |
| 10 | M | POGS | Negative | POGS | PR | Disease free at 55.2 months |
| 12 | M | AML M2 | 65% | Residual disease | PD | Expired at 8.6 months |
| 13 | M | AML M2 | 30% | Residual disease | CR | Expired at 20.2 months |
| 16 | M | AML M4 | 12% | OGS developed before relapse | CR | Alive with disease at 15.8 months |
NA-Not available, ND –not done-male-female, AML-acute myelogenous leukemia, POGS-primary orbital granulocytic sarcoma.
CR-complete response, PR-partial response, SD-stable disease, PD-progressive disease, RT-radiation therapy.
Treatment Details and Outcome
All patients received planned chemotherapy with Daunorubicin (60-90mg/m2 IV day 1 to day 3) and Cytosine Arabinoside (Ara C) (100-200 mg/m2 IV day 1 to day 7). Six patients achieved bone marrow remission after induction chemotherapy. Median prescribed dose was 24.5 Gy (Range: 15-45 Gy). Median follow-up was 24 months (Range 24-84 months). Out of 11 patients, 5 (45.4%) had complete response, 3 (27.27%) had partial response, 1 patient had stable disease (9%) and 2 developed progressive disease (18%). At last follow-up, 7 (63.6%) patients were alive and four patients were dead due to progressive disease elsewhere. During radiotherapy all patients developed grade I conjunctivitis and grade I skin reaction and managed conservatively. Only two patients had grade II conjunctival reaction. Six patients had haematological relapse. Three patients developed spinal metastasis and treated with palliative radiotherapy to a dose of 12 Gy in 3 fractions over 3 days. One patient developed progressive disease in the orbit during radiation therapy and another patient developed progression on follow-up. Hence the 2 year local control was 81.8%. Overall median time for haematological relapse was 3 months. Treatment and outcome details of each patient are summarized in [Table/Fig-4].
Treatment details and clinical outcome.
| Sl No. | Post chemo status in orbit | RT dose | RT technique | Fields used | Prescriptioniso-dose | Response toradiation | Status at last follow-up |
|---|
| 1 | Residual disease | 21.6Gy | 3DCRT | 2 anterior oblique | 90% | SD | Alive at 13.3 months |
| 2 | POGS | 45Gy | 3DCRT | 2 anterior oblique | 91% | CR | Disease free at 75 months |
| 3 | Developed OGS during leukemia treatment | 25Gy | 3DCRT | 2 anterior oblique | 95% | PD | Alive with disease at 18.3 months |
| 4 | Post completion of treatment developed OGS | 24Gy | 3DCRT | 2 anterior oblique | 95% | CR | Disease free at 29.2 months |
| 5 | Residual disease | 30Gy | 3DCRT | 2 anterior oblique | 90% | PR | Died after 16.4months |
| 6 | Residual disease | 25Gy | 3DCRT | 2 anterior oblique | 90% | PR | Died after 10.9 months |
| 7 | OGS developed post completion of treatment | 24Gy | 3DCRT | 2 anterior oblique | 95% | CR | Alive with disease at 8.5 months |
| 8 | POGS | 36Gy | 3DCRT | 2 anterior oblique | 97% | PR | Disease free at 55.2 months |
| 9 | Residual disease | 30Gy | 3DCRT | Anterior, lateral oblique | 90% | PD | Died at 8.6 months |
| 10 | Residual disease | 24Gy | 3DCRT | Anterior, lateral oblique | 95% | CR | Died at 20.2 months |
| 11 | OGS developed before relapse | 15Gy | 3DCRT | Anterior, lateral oblique | 90% | CR | Alive with disease at 15.8 months |
POGS-primary orbital granulocytic sarcoma.
CR-complete response, PR-partial response, SD-stable disease, PD-progressive disease, RT-radiation therapy.
Discussion
This report was aimed to study the role of radiotherapy on the outcome of OGS. Our study demonstrates the beneficial role of radiotherapy as palliation. GS, an uncommon solid tumour comprising of cell of myeloid precursor has been hypothesized to originate in the bone marrow which permeates through the Haversian canal to the sub-periosteum [6]. The soft tissue of head and neck is the most common area to be involved followed by central nervous system and para-spinal area. In the head and neck, orbit is involved most frequently followed by epidural tissue and skull whereas involvement of maxilla, para nasal sinus and nasal cavity has been reported in isolated case reports only [7–9]. It may occur as a separate entity as primary granulocytic sarcoma or secondarily associated with haematological disorders. It is commonly associated with AML and hence known as extra medullary manifestation of AML. Primary GS are known precursors of occurrence of haematological malignancies. In the present study 18% patients had GS without any evidence of pre existing AML whereas rest of the cases (82%) were associated synchronously with AML. This observation concurred with other studies, which reported incidence as high as 16% of isolated GS [10–12].
Proptosis, unilateral or bilateral is the most common presentation reported to affect as high as 100% patients followed by visual complication and pain in 10-15% cases [13,14]. Hence, orbital GS is often considered a diagnostic dilemma and the differential diagnosis includes orbital lymphoma, Primitive Neuroectodermal Tumour (PNET), Ewing’s Sarcoma (ES), rhabdomyosarcoma and metastasis [15]. In paediatric patients retinoblastoma is another differential. Histopathological evaluation and immunohistochemistry (IHC) play pivotal role to differentiate between the various possible diagnoses. OGS is usually immunopositive for MPO, CD15, CD45 and neutrophil elastase whereas orbital lymphoma is usually immunopositive for CD45, B cell markers i.e., CD19, CD 20, CD 22, CD79a and rarely T cell markers i.e., CD3 or Natural Killer (NK) cell marker i.e., CD56 Synaptophysin and Neurofilament Protein (NF) are usually positive in ES/PNET whereas all metastatic carcinoma are positive for cytokeratin [16]. This is of particular importance where GS presents in the absence of any haematological abnormality. In the present series also the patients with a primary GS were biopsied and found to immunonegative for LCA/MPO/MIC2/NSE/Desmin/CD99 /CD20/CD3 /CD68/CD1a and positivity for CD45, CD31, and CD13.
The most common haematological malignancy associated with OGS is AML (AML-M2-60-70%, AML-M4-20%) and chemotherapy is the standard treatment. Induction chemotherapy with a combination of Ara-C and Daunorubicin is used, followed by consolidation with high dose Ara C in patients who achieved complete response [17]. Similar chemotherapy drugs were administered in the present study, complete response was achieved in 55% patients’. However, 9 patients had residual disease. Radiation in the present series was contemplated to achieve an optimal local disease control, improving vision and pain control. Several studies support use of moderate dose of radiation in providing symptom relief, response or disease stabilization in the orbit [18–22]. The role of radiation in OGS has not been highlighted in the literature due to anticipated morbidity. Anticipation of long term toxicity led to limited use of radiation therapy and AAML0531 protocol did not recommend the use of radiation in the absence of gross symptom burden.
Radiation therapy to a dose of 20 Gy has been prescribed in COG protocol 2891 and 2961, however the findings of 2961 trial has been inconclusive. [Table/Fig-5] summarizes the comparison between our cohort and COG cohort. It is noteworthy to mention that inherent radio sensitivity of orbital GS necessitates a moderate dose of radiotherapy (24-30 Gy) and use of conformal radiotherapy in the form of 3DCRT or Intensity Modulated Radiotherapy (IMRT) leads to significant reduction in radiation related acute and late morbidities. There is little doubt that such an approach will impart better quality of life. OGS is essentially one of the manifestations of the underlying systemic haematological disorder and local radiation may not improve overall survival. However, there is evidence that it may improve disease free survival in orbital GS associated with MDS and PV as it is evident in our study [17].
Comparison between COG orbital granulocytic sarcoma patients and our cohort.
| Demographics | COG cohort [17] | Present cohort |
|---|
| Number of patientsGenderMaleFemaleAgeMedian(years)0-23-1011-21 | 231494.79113 | 11837173 |
| Enlarged liver | 6 | 0 |
| Normal liverEnlarged spleenNormal spleenFAB classificationM0M1M2M3M4M5M6M7OtherPOGSBi-phenotypic leukemiaCyto-geneticsNormalt(8,21)abnormal 16abnormal 11t(6,9)(p23q34)-7/7q-5/5q+8+21No dataWBC (10*3micro liter)MedianPlatelets(10*3 micro liter) | 173200011026004000101500000513.190 | –011006020002122NANA |
COG-Cooperative Oncology Group, NA-not available
Limitation
The study has limitations of small sample size and retrospective analysis. However, note should be made that there is very limited literature addressing the effectiveness of radiation in these patients. Anticipation of toxicity and lack of timely referral results to disease progression and loss of vision. The present study highlights the importance of radiation as a useful modality to optimize symptom control for these patients. A well-designed prospective study may be worthwhile to confirm the effectiveness of radiotherapy in GS.
Conclusion
OGS is an uncommon manifestation often associated with AML. Chemotherapy is the cornerstone of management. In patients with residual orbital disease after chemotherapy, low dose radiation can be used to improve local disease control and improve quality of life. Local conformal radiotherapy of 24-30 Gy appears optimal with excellent local control and minimal morbidity. We advocate low dose conformal local radiation in patients of orbital GS who have a poor or partial response to systemic chemotherapy to improve treatment outcome.
NA-Not available, ND –not done-male-female, AML-acute myelogenous leukemia, POGS-primary orbital granulocytic sarcoma.
CR-complete response, PR-partial response, SD-stable disease, PD-progressive disease, RT-radiation therapy.
COG-Cooperative Oncology Group, NA-not available
[1]. Inverardi D, Lazzarino M, Morra E, Bernasconi P, Merante S, Canevari A, Extramedullary disease in Ph’-positive chronic myelogenous leukemia: frequency, clinical features and prognostic significanceHaematologica 1990 75(2):146-48. [Google Scholar]
[2]. Yamauchi K, Yasuda M, Comparison in treatments of non Leukaemic granulocytic sarcoma: report of two cases and a review of 72 cases in the literatureCancer 2002 94(6):1739-46. [Google Scholar]
[3]. Guermazi A, Feger C, Rousselot P, Merad M, Benchaib N, Bourrier P, Granulocytic sarcoma (chloroma): imaging findings in adults and childrenAJR Am J Roentgenol 2002 178(2):319-25. [Google Scholar]
[4]. Yossia S, de Talhoueta S, Ducastelle-Leprêtreb S, Hassounia A, Pignéa G, Selmajia I, Radiotherapy of chloroma or granulocytic sarcoma: A literature reviewCancer/Radiothérapie 2016 20(1):60-65. [Google Scholar]
[5]. Eisenhauer EA, Therasse P, Bogaerts J, Schwartz LH, Sargent D, Ford R, New response evaluation criteria in solid tumours: revised RECIST guideline (version 1.1)Eur J Cancer 2009 45(2):228-47. [Google Scholar]
[6]. Liu PI, Ishimaru T, McGregor DH, Okada H, Steer A, Autopsy study of granulocytic sarcoma (chloroma) in patients with myelogenous leukemia, Hiroshima-Nagasaki 1949-1969Cancer 1973 31(4):948-55. [Google Scholar]
[7]. Ooi GC, Chim CS, Khong PL, Au WY, Lie AK, Tsang KW, Radiologic manifestations of granulocytic sarcoma in adult leukemiaAJR Am J Roentgenol 2001 176(6):1427-31. [Google Scholar]
[8]. Pui MH, Fletcher BD, Langston JW, Granulocytic sarcoma in childhood leukemia: imaging featuresRadiology 1994 190(3):698-702. [Google Scholar]
[9]. Roby BB, Drehner D, Sidman JD, Granulocytic sarcoma of paediatric head and neck: an institutional experienceInt J Paediatr Otorhinolaryngol 2013 77(8):1364-66. [Google Scholar]
[10]. Lee YH, Lee NJ, Choi EJ, Kim JH, Granulocytic sarcoma (chloroma) presenting as a lateral neck mass: initial manifestation of leukemia: a case reportEur Arch Otorhinolaryngol 2006 263(1):16-18. [Google Scholar]
[11]. Lee B, Fatterpekar GM, Kim W, Som PM, Granulocytic sarcoma of the temporal boneAJNR Am J Neuroradiol 2002 23(9):1497-99. [Google Scholar]
[12]. Dinand V, Yadav SP, Grover AK, Bhalla S, Sachdeva A, Orbital myeloid sarcoma presenting as massive proptosisHaematol Oncol Stem Cell Ther 2013 6(1):26-8. [Google Scholar]
[13]. Shields JA, Stopyra GA, Marr BP, Shields CL, Pan W, Eagle RC, Bilateral orbital myeloid sarcoma as initial sign of acute myeloid leukemia: case report and review of the literatureArchives of Ophthalmology 2003 121(1):138-42. [Google Scholar]
[14]. Lan TY, Lin DT, Tien HF, Yang RS, Chen CY, Wu K, Prognostic factors of treatment outcomes in patients with granulocytic sarcomaActa Haematologica 2009 122(4):238-46. [Google Scholar]
[15]. Payne C, Olivero WC, Wang B, Moon SJ, Farahvar A, Chen E, Myeloid sarcoma: a rare case of an orbital mass mimicking orbital pseudotumour requiring neurosurgical interventionCase Rep Neurol Med 2014 2014:395196 [Google Scholar]
[16]. Aggarwal E, Mulay K, Honavar SG, Orbital extra-medullary granulocytic sarcoma: clinicopathologic correlation with immunohistochemical featuresSurv Ophthalmol 2014 59(2):232-35. [Google Scholar]
[17]. Johnston DL, Alonzo TA, Gerbing RB, Lange BJ, Woods WG, Superior outcome of paediatric acute myeloid leukemia patients with orbital and CNS myeloid sarcoma: a report from the Children’s Oncology GroupPaediatr Blood Cancer 2012 58(4):519-24. [Google Scholar]
[18]. Fleckenstein K, Geinitz H, Grosu A, Goetze K, Werner M, Molls M, Irradiation for conjunctival granulocytic sarcomaStrahlenther Onkol 2003 179(3):187-90. [Google Scholar]
[19]. Nishimura S, Kyuma Y, Kamijo A, Maruta A, Isolated recurrence of granulocytic sarcoma manifesting as extra- and intracranial masses–case reportNeurol Med Chir (Tokyo) 2004 44(6):311-16. [Google Scholar]
[20]. Rosenberg C, Finger PT, Furlan L, Iacob CE, Bilateralepibulbar granulocytic sarcomas: a case of an 8-year-old girl with acute myeloid leukemiaGraefes Arch Clin Exp Ophthalmol 2007 245(1):170-72. [Google Scholar]
[21]. Lee SG, Park TS, Cheong JW, Yang WI, Song J, Lee KA, Preceding orbital granulocytic sarcoma in an adult patient with acute myelogenous leukemia with t(8;21): a case study and review of the literatureCancer Genet Cytogenet 2008 185(1):51-54. [Google Scholar]
[22]. Bakst R, Wolden S, Yahalom J, Radiation therapy for chloroma (granulocytic sarcoma)Int J Radiat OncolBiol Phys 2012 82:1816-22. [Google Scholar]