Cardiovascular Disease (CVD) including Coronary Heart Disease (CHD) is the leading cause of death in India [1]. According to the estimated prevalence between the years 1960 to 2000, there is two-fold rise of CHD in rural population and six fold rise in urban population [2]. Coronary Artery Disease (CAD) risk rises progressively with increases in serum cholesterol level and saturated fat intake [3]. However, in Indian population, the role of dietary fat intake and serum cholesterol level in the aetiology of CAD is controversial [4–7].
The rural population in North and East India consumes more rapeseed-mustard oil (Brassica juncea) and grains, which are considered a poor man’s food. In urban areas, Indian ghee and vegetable ghee are substituted for mustard oils and whole grains [8]. Rapeseed-mustard oil contains high amount of erucic acid, varied from 14% to 33% in the lipids [9]. High erucic acid content in foodstuff has been associated with myocardial lipidosis and heart lesions in laboratory rats experiments; therefore not safe for human consumption [10]. Mustard oil also has a high ratio of oleic to linolenic fatty acid and linoleic (ω-6) to linolenic (ω-3) fatty acid [11]. Also, the average Trans Fatty Acid (TFA) content in nonrefined mustard and refined soybean oils is 0.2- to 1.00 g/100g of oil and 0.4 to 1.5g/100g oil, both of which are higher as compared to the limit set by WHO [12]. The limit for human consumption of TFA as a percentage of energy should be less than 1% in fats and oils according to WHO [13].
“Ghee”, the Indian name for clarified butterfat, is usually prepared from cow’s milk, buffalo milk or mixed milk [14]. According to The International Diary Federation (1977), ghee is a product exclusively made from milk, cream or butter from various animal species. During the manufacturing process of ghee, there is almost total removal of moisture (almost anhydrous fat) from the milk and the milk becomes solids-not-fat (ghee), which is only produced in India [15]. Analysis by different groups showed that ghee contains 45-65% saturated fat and 32% Monounsaturated Fatty Acids (MUFA) [16–18]. It is also a good source of lipid nutrients, fat-soluble vitamins and essential fatty acids [17].
Because of its high saturated fat content, consumption of ghee is expected to be associated with high CHD [3]. But, study on healthy young Indian by Shankar et al., indicated that there is no serious adverse effect of ghee on lipoprotein profile [19]. Consuming ghee at the level of 10% of total energy intake in a vegetarian diet generally has no effect on the serum lipid profile of young, healthy, physically active individuals [20]. Similarly, Gupta et al., showed that the prevalence of CHD in men were low, who consumed more ghee in their diet [21].
In our study, we aim to find out the relationship of mustard oil and ghee consumption on CHD history with reference to the relative amount of consumption. We also tried to find the association of CHD history with gender and lipid profile.
Materials and Methods
This cross-sectional study was carried out in Jodhpur city, Rajasthan, India. House-to-house survey was done in such a way that about 15-20 houses were covered in each 5km distance around Dr. S. N. Medical College upto a maximum of 25km from it. The first 5km distance was covered initially, then next 5km and so on. For starting the survey, one house (H0) within 5km radius of Dr. S. N. Medical College was chosen arbitrarily, then 14 to 19 other houses were chosen randomly among all the houses within this 5km radius. After completing house-to-house survey of these houses, the process was repeated for the next 5km, and so on [Table/Fig-1]. The interval distance of 5km, maximum distance of 25km and the number 15-20 for randomly choosing houses at each interval were chosen as per our limitations, convenience and past experience. The survey was done within limited duration of 12months by only one interviewer (one of the authors) with limited working hour. A total of 586 people aged between 40 to 80 years were interviewed during the survey for the amount and type of visible cooking fat consumed in a 24-hour dietary recalls method (the food frequency questionnaire). The Food Frequency Questionnaire (FFQ) is a valid tool for the assessment of dietary habits of Indian subjects [22–24]. The dietary records obtained were subjected to confirmation by trained dieticians of the Institute.
Diagrammatic presentation of house-to-house survey process.
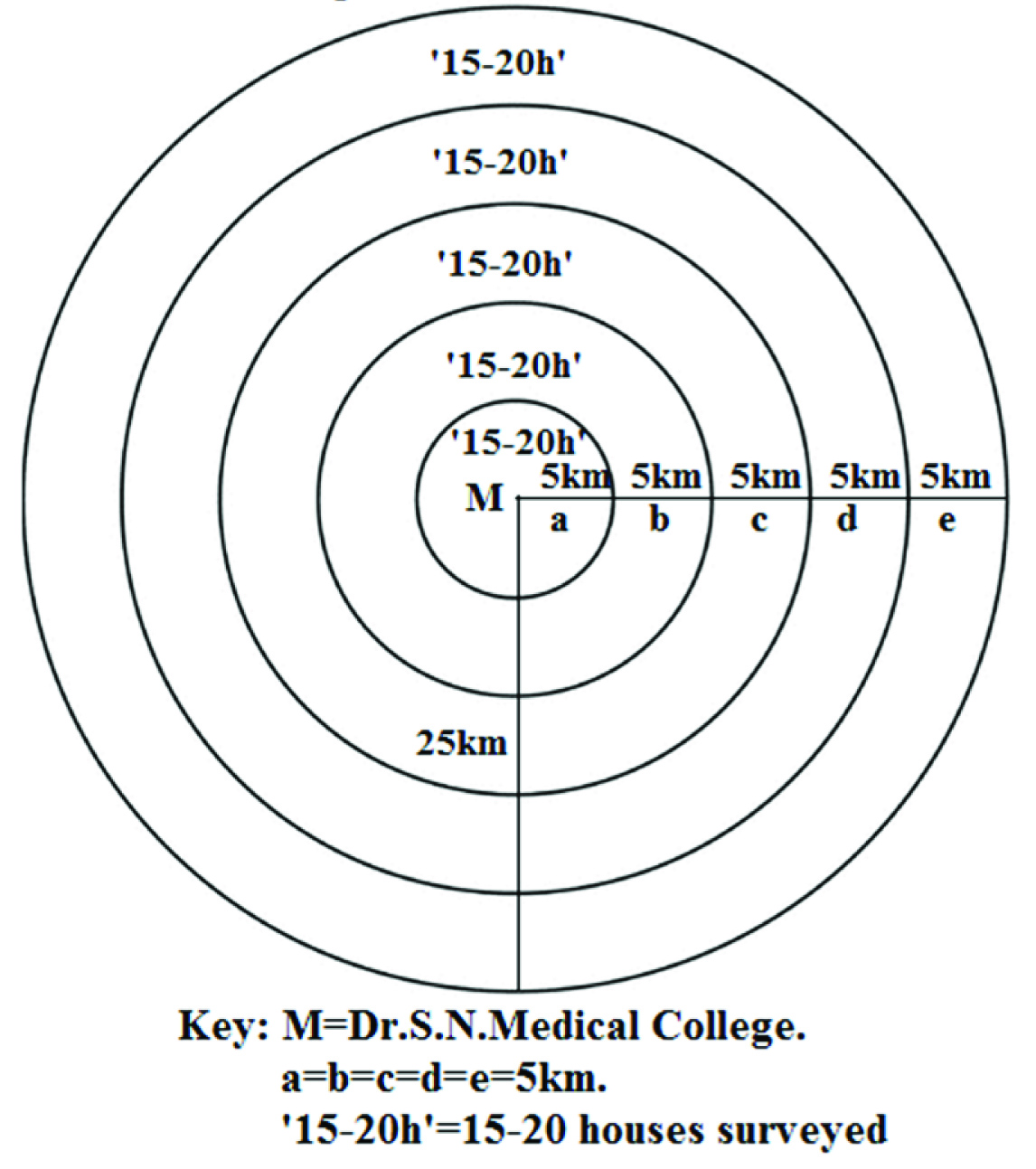
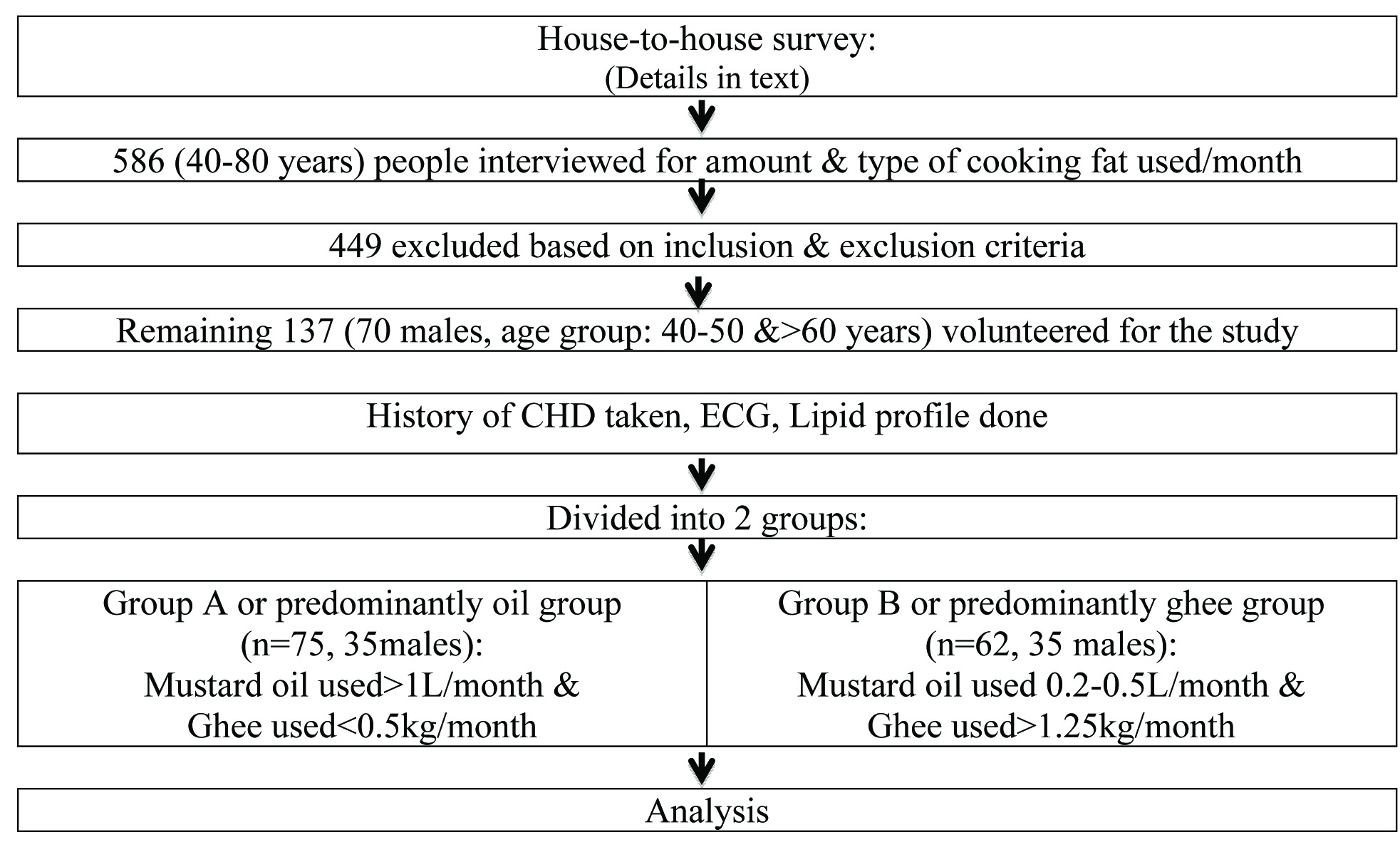
Out of the total population interviewed, 137 people (70 males and 67 females, age group 40-50 years and >60 years) were selected for the study based on the following inclusion and exclusion criteria. Inclusion criteria were: a) age: ≥40 years; b) non-obese (BMI ≤30Kg/m2). Exclusion criteria (risk factor for CHD) were: a) chronic alcoholic; b) chronic smoker; c) history of diabetes mellitus, uncontrolled hypertension, deep vein thrombosis; d) low physical activity or sedentary life style; e) familial history of CHD.
The above information was obtained using a self-report system. History of CHD (documented myocardial infarction or angina) was also recorded by only one interviewer. A well informed and written consent was taken from each participant. Institutional Review Board and Ethical Committee approved the study.
The total study population was evaluated for following laboratory tests during their hospital visit: (a) 8 hour fasting blood samples were collected to determine Lipid profile {Serum TG, Total Cholesterol, LDL Cholesterol, VLDL Cholesterol, HDL Cholesterol, TC/HDL Ratio, LDL/HDL Ratio} by using ELISA, to assess their association with CHD history; (b) Resting ECGs were taken to determine old ischaemic events, which were interpreted by a specialist in the field. CHD history was taken positive if there were ECG findings even in absence of any documentation.
To define the role of mustard oil and ghee on CHD, the average amount consumed in a month was determined and the study population was divided into two groups: Total 75 subjects (35 males and 40 females) consumed mustard oil more than 1L/month, but ghee less than 0.5Kg/month (group A or predominantly oil group) and 62 subjects (35 males and 27 females) consumed ghee >1.25Kg/month but mustard oil 0.2 to 0.5L/month (group B or predominantly ghee group) [Table/Fig-2]. The cutoff value for the group division was taken according to a previous study in Indian population [21].
Statistical Analysis
The comparison of the history of CHD in different age groups, among gender and in groups with different fat consumption per month was done using Pearson Chi-Square test. Fisher’s-Exact test p-value was used, whenever the minimum expected count in any of the cells of contingency tables came to be less than 5. Kendall’s tau b was used to assess the correlation of history of CHD with various variables among the groups. In it, the coding used for analysis was: for CHD history, 0 for negative, 1 for positive; for age group, 1 for 40-50 years and 2 for >60 years; and for type of fat consumed per month,1 for predominantly oil and 2 for predominantly ghee. Binary logistic regression with history of CHD as dependent variable (0=negative and 1=positive), and gender (0=male and 1=female) and different fat consumption group (0=group A and 1=group B) as independent variables was evaluated. Other potential independent variables were not included in the model due to multicollinearity. To assess the best classifier for positive CHD history, areas under the Receiver Operating Characteristic (ROC) curves were calculated for all the potential predictor variables. The cut off values were chosen at the maximum Youden Index (J), where J = sensitivity+specificity-1. SPSS (Statistical Package for Social Science) version 20.0 software was used for data analysis. Statistical significance was chosen at α value of ≤.05 for all the analyses.
Results
In the present study, the maximum frequency of CHD was present among the male subjects, and in each gender group among the 40-50 years age group, although statistically non-significant [Table/Fig-3]. Unfortunately those belonging to age group of 50-60 years did not satisfy inclusion criteria or satisfy exclusion criteria, and hence were not available for the study. Importantly, in both the genders, the maximum frequency of CHD was found in predominantly oil group, which was, however, not statistically significant [Table/Fig-4].
History of CHD in different age groups as per gender, and in the two genders.
| Gender | Historyof CHD | Age Group (in years) | Total | χ2, df(p-value)# | χ2, df(p-value)^ |
|---|
| 40-50 | > 60 |
|---|
| Male (n=70) | Absent | 28 (47.5%) | 31 (52.5%) | 59 (100.0%) | 0.971, 1 (.324) | 0.408, 1 (.523) |
| Present | 7 (63.6%) | 4 (36.4%) | 11 (100.0%) |
| Total | 35 (50.0%) | 35 (50.0%) | 70 (100.0%) |
| Female (n=67) | Absent | 34 (57.6%) | 25 (42.4%) | 59 (100.0%) | 0.884, 1 (.459)^^ |
| Present | 6 (75.0%) | 2 (25.0%) | 8 (100.0%) |
| Total | 40 (59.7%) | 27 (40.3%) | 67 (100.0%) |
*p–value≤0.05: significant; **p–value≤0.01: highly significant. #Pearson Chi-Square test between history of CHD & different age groups as per gender. ^Pearson Chi-Square test between history of CHD & gender. ^^Fisher’s Exact test p-value. χ2= Chi-Square & df=degree of freedom.
History of CHD in groups with different type of fat consumed per month as per gender.
| Gender | History ofCHD | Type of Fat Consumed/Month | Total | χ2, df(p-value) |
|---|
| Predominantly Oil | Predominantly Ghee |
|---|
| Male (n=70) | Absent | 28 (47.5%) | 31 (52.5%) | 59 (100.0%) | 0.971, 1 (0.324) |
| Present | 7 (63.6%) | 4 (36.4%) | 11 (100.0%) |
| Total | 35 (50.0%) | 35 (50.0%) | 70 (100.0%) |
| Female (n=67) | Absent | 34 (57.6%) | 25 (42.4%) | 59 (100.0%) | 0.884, 1 (0.459)^^ |
| Present | 6 (75.0%) | 2 (25.0%) | 8 (100.0%) |
| Total | 40 (59.7%) | 27 (40.3%) | 67 (100.0%) |
*p–value≤0.05: significant; **p–value≤0.01: highly significant. Pearson Chi-Square test.^^Fisher’s Exact test p-value. χ2= Chi-Square & df=degree of freedom.
There was a significant positive correlation between the history of CHD with TG, TC, LDL, VLDL, TC/HDL and LDL/HDL with the exception of HDL that showed significant negative correlation [Table/Fig-5]. Interestingly, the total mustard oil consumed per month showed positive correlation with the history of CHD, which was also significant among the female subjects [Table/Fig-5]. This indicated that among the studied subjects, especially among the female subjects, who consumed more mustard oil per month, the frequency of CHD was more. This however didn’t indicate cause and effect relationship.
Correlation of history of CHD with various variables as per gender.
| Variables | Age Group(in years)^(M, F) | Type of FatConsumedper month^^(M, F) | Total OilConsumed(L/month)(M, F) | Total GheeConsumed(Kg/month)(M, F) | TG (mg/dl)(M, F) | TC (mg/dl)(M, F) | HDL (mg/dl)(M, F) | LDL (mg/dl)(M, F) | VLDL (mg/dl)(M, F) | TC/HDL(M, F) | LDL/HDL(M, F) |
|---|
| History of CHD# | (-0.118, -0.115) | (-0.118, -0.115) | (0.214, 0.267*) | (-0.188, -0.142) | (0.286**, 0.206*), | (0.466**, 0.421**) | (-.452**, -0.388**) | (0.468**, 0.434**) | (0.408**, 0.212*) | (0.485**, 0.442**) | (0.495**, 0.453**) |
*p–value≤0.05: significant; **p–value≤0.01: highly significant. Kendall’s tau b (r-value given). #History of CHD, 0=Absent & 1=Present; ^Age Group,1=40-50 & 2=>60; and ^ Type of Fat Consumed per Month,1=Predominantly Oil, 2=Predominantly Ghee. M=Male & F=Female.
Also, the odds of CHD among the predominantly ghee group was 0.491 times that of predominantly mustard oil group, which was adjusted for the difference in gender [Table/Fig-6]. This indicated that the odds of CHD were higher by 50.9% among the predominantly mustard oil group as compared to the predominantly ghee group, and this was independent of gender. The odds was higher also among the male subjects by 32.2% (odds ratio or OR= 0.678), independent of the type of fat consumption per month [Table/Fig-6]. However, the findings were not statistically significant.
Logistic regression model for history of CHD and type of fat consumption per month.
| Variables | B | p-value | Exp(B) | 95% C.I. for Exp(B) | Hosmer andLemeshow testχ2, df (p-value) | Overallpercentagecorrectlypredicted |
|---|
| Lower | Upper |
|---|
| ^Gender (1) | -0.389 | 0.442 | 0.678 | 0.251 | 1.826 | 0.014, 2 (.993) | 86.1% |
| #Group (1) | -0.712 | 0.180 | 0.491 | 0.173 | 1.389 |
| Constant | -1.367** | 0.000 | 0.255 | |
*p–value≤0.05: significant; **p–value≤0.01: highly significant. ^Gender, 0=Male (reference) & 1=Female; and #Group, 0=Predominantly Oil (reference) & 1= Predominantly Ghee. B=beta weights or regression coefficients, Exp(B)=eB, C.I.=Confidence Interval, χ2= Chi-Square & df=degree of freedom.
Among the statistically significant AUCs, that of LDL/HDL were the largest, indicating it to be the best classifier for positive CHD history in both the gender [Table/Fig-7]. The other statistically significant classifiers with corresponding cutoff values at maximum J for positive CHD history were given in [Table/Fig-5]. It is to be noted that the total mustard oil consumed per month was a statistically significant classifier for positive CHD history among the female subjects, and when the all the studied subjects were analysed as a whole [Table/Fig-7]. The cut off values obtained were ≥2.055 L/month for the female subjects, and ≥1.745 L/month when all the subjects were analysed as a whole [Table/Fig-7].
Area under the ROC Curves of the variables for the prediction of having CHD as per gender.
| Gender | Variables | AUC | S.E. | p-value | Cut-offvalue atmaximum J |
|---|
| Male (n=70) | LDL/HDL | .976** | .022 | .000 | ≥4.97 |
| TC/HDL | .968** | .021 | .000 | ≥7.14 |
| LDL (mg/dl) | .949** | .027 | .000 | ≥171 |
| TC (mg/dl) | .944** | .027 | .000 | ≥242.5 |
| HDL (mg/dl) | .925** | .032 | .000 | ≤35.5 |
| VLDL (mg/dl) | .888** | .041 | .000 | ≥39.5 |
| TG (mg/dl) | .773** | .070 | .004 | ≥105 |
| Total Oil Consumed (L/month) | .672 | .103 | .072 | ≥1.745 |
| Total Ghee Consumed (Kg/month) | .650 | .073 | .116 | ≤1.435 |
| Female (n=67) | LDL/HDL | .989** | .010 | .000 | ≥5.01 |
| TC/HDL | .977** | .018 | .000 | ≥6.755 |
| LDL (mg/dl) | .968** | .020 | .000 | ≥154.5 |
| TC (mg/dl) | .948** | .028 | .000 | ≥222 |
| HDL (mg/dl) | .910** | .047 | .000 | ≤31.5 |
| Total Oil Consumed (L/month) | .748* | .118 | .024 | ≥2.055 |
| VLDL (mg/dl) | .725* | .108 | .040 | ≥35.5 |
| TG (mg/dl) | .720* | .080 | .044 | ≥99.5 |
| Total Ghee Consumed (Kg/month) | .620 | .089 | .275 | ≤1.435 |
| Combined (n=137) | LDL/HDL | .981** | .014 | .000 | ≥4.97 |
| TC/HDL | .972** | .014 | .000 | ≥6.755 |
| LDL (mg/dl) | .957** | .017 | .000 | ≥154.5 |
| TC (mg/dl) | .936** | .021 | .000 | ≥222 |
| HDL (mg/dl) | .885** | .030 | .000 | ≤35.5 |
| VLDL (mg/dl) | .823** | .055 | .000 | ≥35.5 |
| TG (mg/dl) | .750** | .052 | .000 | ≥99.5 |
| Total Oil Consumed (L/month) | .699** | .078 | .005 | ≥1.745 |
| Total Ghee Consumed (Kg/month) | .630 | .057 | .069 | ≤1.435 |
*p–value≤0.05: significant; **p–value≤0.01: highly significant. Receiver Operating Characteristic (ROC) curve. AUC=Area under the ROC Curve, S.E.=Standard Error, and J=Youden Index (J=Sensitivity+Specificity-1).
Hence, our study indicated the negative effect of increased mustard oil consumption in the form of increased positive CHD history, and suggested that total mustard oil consumption should be less than 1.75-2.06 L/month.
Discussion
The data of the present study indicates, CHD history positively correlated with serum TG, TC, LDL, VLDL, TC/HDL and LDL/HDL and negatively correlated with serum HDL. There are many evidences which support that an elevated LDL-C concentration in plasma is atherogenic [25,26], whereas a high HDL-C level is cardioprotective [27,28]. Furthermore, study by Assmann et al., confirmed TG as an independent risk factor of CHD, irrespective of serum LDL-C and HDL-C level [29]. High TC/HDL cholesterol ratio and LDL /HDL ratio are also indicator of ischemic heart disease risk in men [30,31].
The data of our present study also indicates that male persons who were consuming mustard oil were more prone to CHD history than ghee. The adverse effect of mustard oil consumption on CHD may be due to the following reasons:
First, the rapeseed-mustard oil contains high oleic acid and low linolenic acid [11]. Low dietary linolenic acid intake associated with high risk of ischaemic heart disease had been reported in several prospective studies, including the Multiple Risk Factor Intervention Trial [32], the Health Professionals’ Follow-up Study [33] and the Nurses’ Health Study [34].
Second, rapeseed-mustard oil also contains very high level of erucic acid (C22:1) [9]. There are reports that erucic acid causes myocardial lipolysis [10,35]. The effect of erucic acid may compromise some of the beneficial effect of the linoleic acid in mustard oil. This may be the reason of high CHD history in the mustard oil group compared to ghee group in our study.
Many researches had already reported the beneficial properties of ghee in the form of decrease in serum total cholesterol, LDL, VLDL, and triglycerides; decreased liver total cholesterol, triglycerides, and cholesterol esters. In animal study, on rats also showed no effect of 5% and 10% ghee-supplemented diets on serum cholesterol and triglycerides. When the ghee level in foodstuffs was less than 10%, it did not increase liver microsomal lipid peroxidation or liver microsomal lipid peroxide levels [36]. In Wistar rats model, Kumar et al., showed ghee has hypocholesterolaemic effect due to significant increased biliary excretion of cholesterol [37]. The negative association of CHD history with ghee consumption, although statistically non-significant, is thus understandable.
Randomized controlled trial on healthy young Indians, Shankar et al., showed ghee has no significant effect on the serum lipid profile when it is consumed in <10% of total energy intake as compared to mustard oil [19]. Another study by the same group showed that introducing ghee as a partial replacement for mustard oil leads to rise in TC as well as HDL-C levels, so no significant change occurs in TC/HDL-C ratio. They concluded that the rise in HDL cholesterol might be due to the considerable MUFA content of ghee [20]. Recent meta-analysis by Chowdhury et al., clearly showed that high consumption of polyunsaturated fatty acids and low consumption of total saturated fats are not the cadrioprotective diet for coronary diseases [38].
In addition to the above literature, our study is also supported by a similar study on rural population of Rajasthan, India by Gupta et al., which showed a significantly lower prevalence of CHD in men who consumed higher amounts of ghee more than 1kg/month. Multivariate analysis confirmed this association (p< 0.001) [21].
So, the available data in the literature do not support a conclusion of harmful effects of the moderate consumption of ghee in the general population, although it contains high level of saturated fat. On the other hand the harmful effects of mustard oil are more on CHD and may be due to its high erucic acid content. Raheja also had pointed out this fact, that although Asian Indians were using ghee in their cooking for generations, there was a low incidence of CHD. Three to four decade ago, when the traditional fat had been replaced by oils rich in linoleic and arachidonic acid [39,40], as well as trans fatty acids which comprise 40% of vanaspati [41], the epidemic of CHD in India had begun. Adulteration of commercially prepared ghee with vanaspati is also a common practice in India. Because of this, researchers investigating ghee should be cautious and ensure that the ghee used in their experiments is pure and not adulterated with vanaspati, which could yield spurious results.
Limitation
Our study was a preliminary survey to find out the association of CHD with relative amount of mustard oil and ghee consumption. Study was having several limitations. Firstly, the major limitation of our study was very small number of sample size. Further studies, preferably well designed, blinded, randomized, controlled, prospective studies, are required on a sufficiently large sample size, from different strata of society and different region of India, for the evaluation of effect of mustard oil or ghee consumption on CHD. Secondly, it was just a survey, and not an experimental or interventional controlled prospective cohort study. Thirdly, to ascertain the fat intake we relied on the words of the subject, which could result in recall and other type of bias.
Conclusion
The present study suggests that increased mustard oil consumption had adverse effect in the form of increased positive CHD history, and the adverse opinion about ghee on CHD in the medical community may not be valid as compared to mustard oil. However, extrapolation of this result on the entire population should be done cautiously because the subjects of this study were small in number and subjects were selected from only one geographical region. Further well-designed studies are required on a larger number of subjects, from different regions of India.
*p–value≤0.05: significant; **p–value≤0.01: highly significant. #Pearson Chi-Square test between history of CHD & different age groups as per gender. ^Pearson Chi-Square test between history of CHD & gender. ^^Fisher’s Exact test p-value. χ2= Chi-Square & df=degree of freedom.
*p–value≤0.05: significant; **p–value≤0.01: highly significant. Pearson Chi-Square test.^^Fisher’s Exact test p-value. χ2= Chi-Square & df=degree of freedom.
*p–value≤0.05: significant; **p–value≤0.01: highly significant. Kendall’s tau b (r-value given). #History of CHD, 0=Absent & 1=Present; ^Age Group,1=40-50 & 2=>60; and ^ Type of Fat Consumed per Month,1=Predominantly Oil, 2=Predominantly Ghee. M=Male & F=Female.
*p–value≤0.05: significant; **p–value≤0.01: highly significant. ^Gender, 0=Male (reference) & 1=Female; and #Group, 0=Predominantly Oil (reference) & 1= Predominantly Ghee. B=beta weights or regression coefficients, Exp(B)=eB, C.I.=Confidence Interval, χ2= Chi-Square & df=degree of freedom.
*p–value≤0.05: significant; **p–value≤0.01: highly significant. Receiver Operating Characteristic (ROC) curve. AUC=Area under the ROC Curve, S.E.=Standard Error, and J=Youden Index (J=Sensitivity+Specificity-1).