Omphalitis is the infection of the umbilical cord stump, which can lead to septicaemia and significant neonatal morbidity and mortality [1]. Omphalitis caused many neonatal deaths, where aseptic delivery techniques were not used [2]. Clinical criteria for defining omphalitis has varied considerably in literatures published across the world, ranging from discharge of pus with/without foul odour, redness, swelling, warmth and tenderness [3]. However, an algorithm for the objective diagnosis of omphalitis suggested by researchers from Nepal on the basis of presence of pus and moderate redness or severe redness for inclusion of cases and an absence of pus along with no moderate/severe redness/swelling for exclusion, were found to be the most pragmatic clinical screening criteria [3]. Omphalitis is a significant risk factor for positive blood cultures in Neonatal Intensive Care Unit (NICU) [4]. Patent vitello-intestinal duct, umbilical granuloma, umbilical sinus and patent urachus are the other causes of umbilical discharge [5], which should also be ruled out.
Materials and Methods
A prospective study was done after obtaining ethical clearance from the Institutional Ethics Committee. Neonates (≤ 28 days of age) were screened for omphalitis during a period of four months (from 1st January 2016 to 30th April 2016). A total of 623 neonates born in our institute, were screened for omphalitis in the inpatient and outpatient department of Paediatrics, using the clinical criteria as suggested by researchers from Nepal [3]. Following the clinical examination, umbilical discharge from the included cases was collected with sterile cotton swab stick and immediately inoculated on blood agar, MacConkey’s agar and brain heart infusion broth with a simultaneous direct Gram stain smear. The inoculated media were incubated at 37°C and kept for 48 hours, readings being taken after 24 hours and 48 hours. Grown organisms were identified by standard microbiological techniques including Gram stain, relevant biochemical reactions and VITEK 2 Compact (bioMereiux Inc., France). Antimicrobial susceptibility testing was performed on Mueller Hinton agar plates by Kirby Bauer disc diffusion method and interpretation was done according to the Clinical and Laboratory Standards Institute (CLSI) guidelines version 2015 using American type culture collection strain of Staphylococcus aureus (ATCC 25923) for quality control. The Staphylococcus isolates were tested with cefoxitin (30μg), ciprofloxacin (5μg), erythromycin (15μg), clindamycin (2μg), trimethoprim-sulphamethoxazole (1.25/23.75μg), amikacin (30μg), gentamicin (10μg), doxycycline (30μg) and linezolid (30μg), by disc diffusion method and vancomycin and teicoplanin by E-strip method according to the CLSI guidelines [6]. For determination of inducible clindamycin resistance in all the Staphylococcus isolates, D-test was performed with erythromycin and clindamycin discs [6].
During the same period of four months growth of MRSA was compared between umbilical discharge and other different pus samples received from skin and soft tissue infections (SSTI) from the neonates. All data were entered in excel spreadsheet (Microsoft Office, Redmond, Washington, USA). The data were summarised using mean along with standard deviation for continuous variables, and frequency along with percentages for categorical variable. Chi-square test was used to check the categorical variables association and p-value <0.05 was taken as significant.
Results
Among the 623 neonates, 21 (3.37%) were positive for our screening criteria for omphalitis. The age of the babies were between 2 days and 28 days (mean = 10.99 days, SD = 7.90). Among the clinically included omphalitis cases, 15 (71.42%) were male and 6 (28.57%) female. Among the cases, 11 (52.38%) were actually discharged post-delivery with apparently healthy stump and presented to the outpatient department with umbilical discharge and redness, 5 (23.81%) were from the paediatric sick neonatal ward and 5 (23.81%) were from NICU.
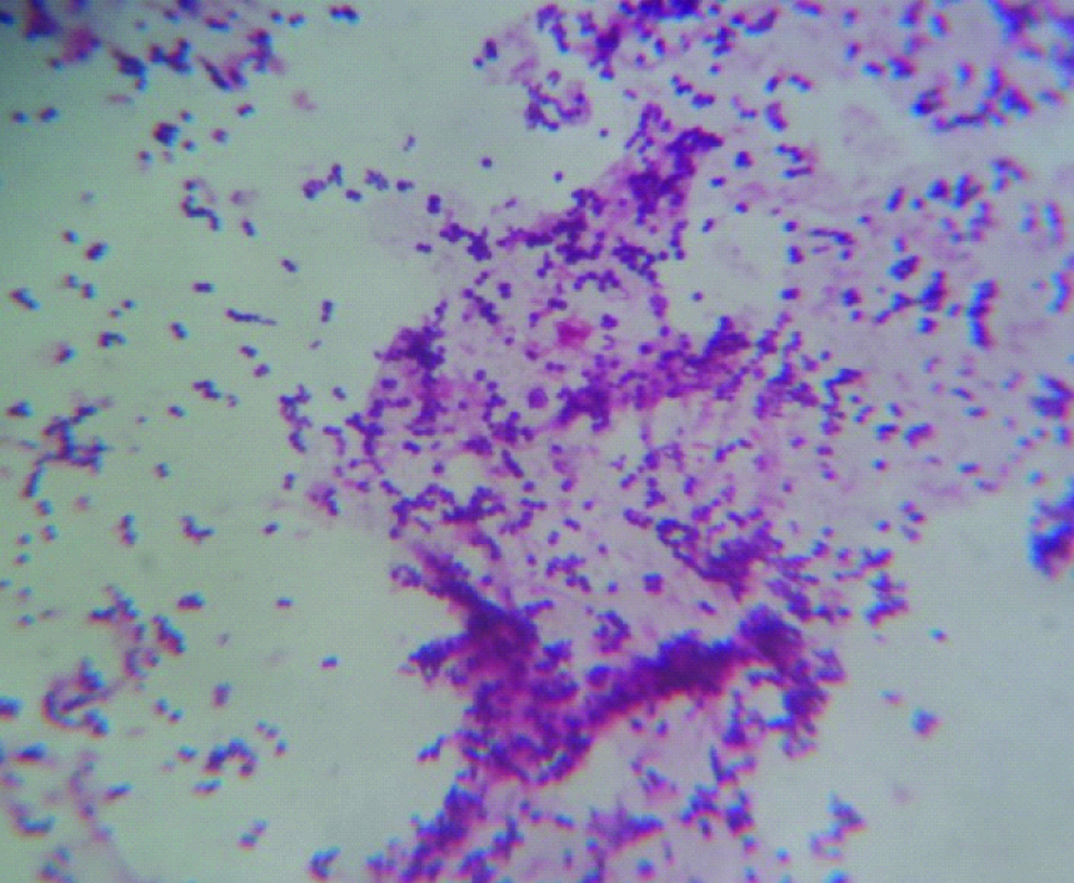
Of these 21 samples, 19 (90.47%) showed plenty of Gram positive cocci in clusters along with polymorphonuclear cells in direct Gram staining [Table/Fig-1] and yielded pure and heavy growth of Staphylococcus aureus after 24 hours incubation. All these 19 isolates (100%) were resistant to cefoxitin and were therefore methicillin resistant Staphylococcus aureus (MRSA). One sample had no growth of any organism and one sample showed growth of commensal cutaneous flora including a mixture of Coagulase negative staphylococci and Corynebacterium spp.
Gram positive cocci in clusters along with polymorphonuclear cells in direct Gram staining.
Out of 24 other different pus samples received from skin and soft tissue infections (SSTI) from the neonates, only 5 (20.83%) had growth of Staphylococcus aureus (MRSA). Hence, the yield of MRSA in umbilical discharge is significantly higher in comparison to that of its yield from any other pus samples from SSTI in neonatal patients (p = 0.000003 ie <0.05, chi-square - 21.8256).
All the isolates of Staphylococcus aureus were susceptible to gentamicin, amikacin, doxycycline, linezolid, vancomycin and teicoplanin and resistant to cefoxitin and ciprofloxacin. All the 6 clindamycin resistant isolates were D-test positive and had inducible clindamycin resistance [Table/Fig-2]
Antimicrobial susceptibility in the Staphylococcus aureus isolates (n=19).
| Antimicrobial drug | Susceptible (%) | Intermediately susceptible (%) | Resistant (%) |
|---|
| Cefoxitin | None | - | 19 (100%) |
| Ciprofloxacin | None | - | 19 (100%) |
| Trimethoprim-sulphamethoxazole | 7 (36.82%) | 8 (42.1%) | 4 (21.05%) |
| Erythromycin | 11 (57.85%) | 1 (5.26%) | 7 (36.82%) |
| Clindamycin | 13 (68.42%) | - | 6 (31.57%) |
| Doxycycline | 19 (100%) | - | None |
| Gentamicin | 19 (100%) | - | None |
| Amikacin | 19 (100%) | - | None |
| Linezolid | 19 (100%) | - | None |
| Vancomycin | 19 (100%) | - | None |
| Teicoplanin | 19 (100%) | - | None |
Discussion
Methicillin resistant Staphylococcus aureus (MRSA) is a serious public health concern and an economic burden to national health care systems [7]. Methicillin resistant Staphylococcus aureus is resistant to all beta-lactam antibiotics including beta-lactam/beta-lactamase inhibitor combinations, cephalosporins, and carbapenems. MRSA has been associated with a variety of healthcare associated as well as community acquired infections including septicaemia, pneumonia, wound infection, septic arthritis, osteomyelitis and toxic shock syndrome with significant rates of morbidity and mortality [8].
In a study from Tanzania, Staphylococcus aureus was the most predominant isolate from SSTI pus swabs in all neonates [9]. In a study from Pakistan the common causative agents of omphalitis were found to be Staphylococcus aureus (52.1%) with a predominance (95.7%) of methicillin sensitive Staphylococcus aureus (MSSA), followed by Streptococcus pyogenes (18%), Group B beta-haemolytic streptococci (10 %), Pseudomonas spp., Aeromonas spp. and Klebsiella spp. [10]. In another study from Oman, Staphylococcus aureus was the most common pathogen isolated from neonatal omphalitis, followed by Escherichia coli and Klebsiella spp. [11]. Guvenc et al., from Turkey reported that Staphylococcus aureus and Escherichia coli were the most frequent microorganisms isolated from neonatal omphalitis [12]. Similarly, in the present study, it was found that 90.47% had pure and heavy growth of Staphylococcus aureus and all of them were MRSA, which is a unique observation.
In a study on neonatal sepsis in a tertiary care centre in North India, Staphylococcus aureus (47.3%) was the most common isolated organism followed by Klebsiella pneumoniae and Acinetobacter spp. with high resistance to oxacillin (48.5%) but good sensitivity to aminoglycosides, vancomycin and linezolid among S. aureus isolates [13]. Though in the present study, prevalence of MRSA was higher among S. aureus isolates, which were otherwise all susceptible to aminoglycosides, vancomycin and linezolid.
Limitations
The limitations of this study were the limited number of cases of omphalitis in the hospital which can be overcome by doing larger community based studies, where prevalence of omphalitis may be higher due to home based delivery. There can also be seasonal variation of pathogen, in a tropical country like that of India, which may be overcome by longer duration studies spread across over the seasons.
Conclusion
We hereby report a high prevalence of MRSA in omphalitis among neonates. MRSA isolates are constitutionally resistant to all beta lactam antibiotics, which are by far the safest group of antibiotics used among the neonates and children. The MRSA isolates as reported by other investigators were also significantly resistant to other safe group of antibiotics for the neonatal/paediatric age group, i.e., macrolides and trimethoprim-sulphamethoxazole, thus making the empirical treatment choices difficult, warranting microbiological diagnosis in all cases of omphalitis.