Primary Salivary Gland Malignancy of Trachea: A Clinical Masquerader
Monika Singh1, Megha Sharma2, Minakshi Bhardwaj3, Prajwala Gupta4, Arvind Ahuja5
1 Senior Resident, Department of Pathology, Dr RML Hospital and PGIMER, New Delhi, India.
2 Junior Resident, Department of Pathology, Dr RML Hospital and PGIMER, New Delhi, India.
3 Professor, Department of Pathology, Dr RML Hospital and PGIMER, New Delhi, India.
4 Associate Professor, Department of Pathology, Dr RML Hospital and PGIMER, New Delhi, India.
5 Associate Professor, Department of Pathology, Dr RML Hospital and PGIMERNew Delhi, India.
NAME, ADDRESS, E-MAIL ID OF THE CORRESPONDING AUTHOR: Dr. Monika Singh, Department of Pathology, PGIMER, Dr RML Hospital, New Delhi-110001, India.
E-mail: mona.nov10@gmail.com
Primary tracheal malignancies are rare and present with non specific symptoms hence delaying the diagnosis. A 41-year-old male presented with repeated paroxysmal episodes of breathlessness for which he was being treated with bronchodilators and steroids. Computed Tomography (CT) chest was done revealing a small polypoidal mass lesion arising from lower trachea/carina. On fibre optic bronchoscopy an infiltrative growth was seen at the lower end of trachea following which biopsy was obtained. On histopathologic examination a diagnosis of primary adenoid cystic carcinoma was made. It was concluded that in a case of refractory obstructive pulmonary disease, primary tracheal tumours should be considered as an important differential diagnosis. CT chest, bronchoscopy and biopsy play a vital role in making an accurate diagnosis of such a clinical masquerader.
Adenoid cystic carcinoma, Chronic obstructive pulmonary disease, Primary tracheal malignancies
Case Report
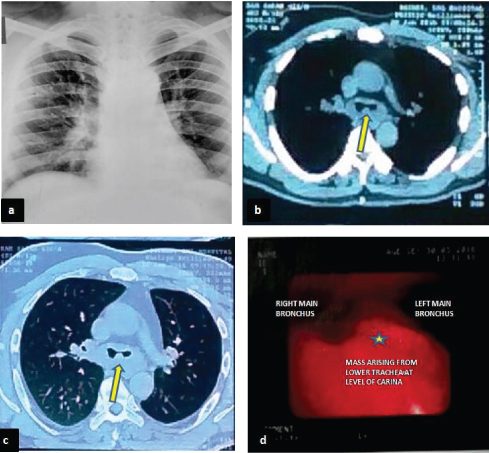
A 41-year-old male presented to the medical emergency with a paroxysmal episode of shortness of breath. Patient was treated with steroids and bronchodilators. Later he was referred to the department of pulmonary medicine. Detailed clinical history revealed that the patient was having similar attacks since last 2 years. In the interval periods of these attacks there was history of cough with on and off haemoptysis. He attended few local hospitals for the same complaints, and was being treated as a case of Chronic Obstructive Pulmonary Disorder (COPD). He was not relieved by the treatment given. Patient was a non smoker and there was no history of tuberculosis or its exposure. General physical examination did not reveal any significant finding. Following this a routine laboratory work up including complete blood count and chest X-ray were done which were within normal limits [Table/Fig-1a]. On spirometric examination, flow volume curve suggested a fixed airway obstruction. Forced Volume Capacity (FVC) was 71% of predicted, Forced Expiratory Volume in 1 second (FEV1) was 40% of predicted and FEV1/FVC was 56% of predicted. CT chest revealed a small polypoidal mass lesion at the level of lower trachea/carina arising from the posterior wall and mild stenosis of the airway lumen at the same level. Distal lung parenchyma however, displayed normal bronchovascular markings. No areas of calcification/necrosis or abnormal enhancement were seen within the mass [Table/Fig-1b,c]. Provisional diagnosis of atypical carcinoid was suggested and fibre optic bronchoscopy followed by biopsy was advised for definitive diagnosis.
Chest X-Ray PA view (a), computed tomography (CT) chest in medisatinal (b) and lung windows(c) and bronchoscopic images. Chest X-Ray is unremarkable while a small isodense polypoidal mass is seen arising from lower trachea at the level of carina (arrow in ‘b’ and ‘c’). Findings confirmed with bronchoscopy displaying the mass at the same location (in‘d’).
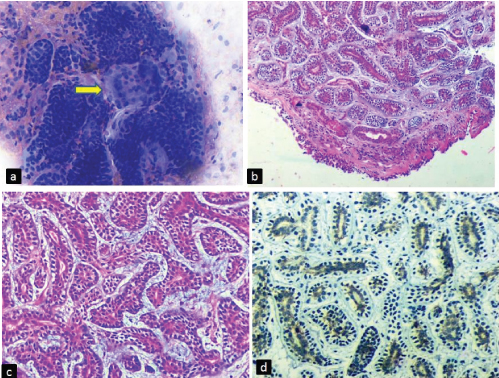
On bronchoscopy, an infiltrative growth at the lower end of trachea was noticed and a provisional diagnosis of tracheal carcinoma was made (Table/Fig-1d). Bronchial brush smears followed by bronchoscopic biopsy was obtained and sent for examination. Brush smears were cellular and were composed of clusters, cords and cup shaped fragments of tumour cells. Many hyaline globules of varying size with adherent tumour cells were also noted. The cells were minimally pleomorphic with round to oval hyperchromatic nuclei, coarse chromatin and scant cytoplasm [Table/Fig-2a]. These cytological features were suggestive of adenoid cystic carcinoma. The cytological findings were confirmed on the biopsy which revealed normal peudostratified respiratory epithelium with underlying tumour cells arranged predominatly in tubular pattern, with areas of cribriform and cord like arrangement in a myxoid matrix. These tubules were lined by intraluminal basaloid cells with hyperchromatic nucleus and moderate amount of cytoplasm and abluminal myoepithelial cells with clear cytoplasm. No mitosis or necrosis was noted in the tumour tissue [Table/Fig-2b,c]. On immunohistochemistry (IHC) the tumour cells were positive for CD117 [Table/Fig-2d]. A final diagnosis of Adenoid cystic carcinoma was given. The patient was referred to a higher centre for further management.
(a) Bronchial brush smear showing clusters, cords and cup shaped fragments of tumour cells. Few hyaline globules (arrow) of varying size with adherent tumour cells are also seen (Giemsa, x400). Histopathology showing (b) peudostratified respiratory epithelium with underlying tumour (H and E, x40). (c) Tumour cells arranged predominantly in tubular pattern, with areas of cribriform and cord like arrangement in a myxoid matrix (H and E, x400). (d) CD117 positive in tumour cells (IHC, x400).
Discussion
Primary salivary gland tumours account for only 1% of all pulmonary neoplasms [1]. Primary tracheal tumours are rare and comprise about 0.1% of respiratory tract neoplasms and less than 1% of all malignancies [1,2]. The incidence of primary tracheal tumour is 2.7 new cases per million /year [3]. Most common primary tracheal malignancy is squamous cell carcinoma (SCC) followed by Adenoid Cystic Carcinoma (ACC). SCC has strong association with history of smoking unlike ACC [4].
ACC was first described by Billroth in 1853 as cylindroma, while the current nomenclature was done by Spies in 1930 [5]. ACC is known for its slow growth rate, with significant interval between presentation and time of diagnosis [6]. ACC usually presents in 5th to 6th decade, without any sex predilection [1].
As mentioned earlier, ACC is a slow growing neoplasm and in most of the cases goes unnoticed until it causes symptoms by infiltrating the adjoining structure depending upon its location. Lung is the most common site for distant metastasis followed by bone, liver and brain [6].
ACC of trachea mostly presents as an intraluminal growth and has a tendency to mimic obstructive pulmonary disorders, as in our case. The location of the tumour in trachea has an impact on the prognosis. Distally placed tumours have a poorer prognosis than the proximal ones. ACC in trachea arises from the seromucinous glands showing myoepithelial and ductal cell differentiation and hence showing reactivity for pan cytokeratin, vimentin, actin, S-100 and CD117 [1].
ACC has three distinct growth patterns: cribriform, tubular and solid with cribriform pattern being the most common type. Despite of heterogeneous growth patterns, cytological homogeneity is seen in the tumour cells. These tumour cells are basaloid, with hyperchromatic nucleus and scant eosinophilic to clear cytoplasm. The tumour cells show either myoepithelial or intercalated duct cell differentiation [6].
Szanto et al., have introduced a grading system based on the percentage of the type of patterns [7]. Grade I, tumours contain exclusively tubular or cribriform patterns, Grade II, tumours have less than 30% solid component in addition to the cribriform or tubular patterns, Grade III, tumours comprise more than 30% solid component.
Several studies on the prognostic predictors of ACC have shown that the presence of solid component is associated with poorer prognosis. However, Kokemueller et al., in a retrospective study of 20 years of ACC in head and neck region concluded that the histological grading system showed inconsistent results as a prognostic marker [6].
In a study of tracheobronchial ACC by Nomori et al., a correlation was noted in histological grade of the tumour with the growth pattern and the prognosis. It was observed that grade I tumours (tubular or cribriform) have an entirely intraluminal growth pattern with better prognosis, while grade III tumours (with >30% solid component) follow predominantly an extraluminal growth pattern and higher incidence of metastasis [8].
ACC can spread via direct extension, submucosal or perineural invasion, and hematogenous route. Though ACC is a gradually growing tumour, however, when associated with distant metastases, survival rate decreases significantly [9].
Approach of surgical resection depends on the location of the tumour in the airway. Surgical resection with reconstruction is the optimal management in most of the cases. However, Calazada et al., in a 30 year study of ACC of airway noted that, surgical resection followed by post-operative adjuvant radiotherapy had a better prognosis and hence a preferred mode of treatment [10]. The 5 year survival rate of patients with tracheal ACC is 52% to 79% and 10 year survival rate is 27% to 51% regardless of the treatment [1].
Conclusion
Primary tracheal malignancies are rare and as they present as an intraluminal growth, often mimicking COPD. ACC of trachea is a slow growing tumour having non specific clinical symptoms and mostly misdiagnosed as COPD at initial stages. This delays the diagnosis and optimal management of the patient and also increases the chance of tumour spread at the time of diagnosis. Hence, it is very crucial to diagnose ACC at the initial stage as once it metastasizes the survival rate falls significantly. This case report highlights the significance of various histological patterns and diagnostic modalities in making an accurate diagnosis of primary tracheal ACC at an early stage.
[1]. Desai HM, Thakare R, Amonkar GP, Karkhanis V, Joshi JM, Adenoid cystic carcinoma of the tracheaIndian J Pathol Microbiol 2015 58:516-18. [Google Scholar]
[2]. Allen MS, Malignant tracheal tumorsMayo Clin Proc 1993 68:680-84. [Google Scholar]
[3]. Azar T, Abdul-Karim FW, Tucker HM, Adenoid cystic carcinoma of the tracheaLaryngoscope 1998 108:1297-300. [Google Scholar]
[4]. Yang KY, Chen YM, Huang MH, Perng RP, Revisit of primary malignant neoplasms of the trachea: Clinical characteristics and survival analysisJpn J Clin Oncol 1997 27:305-09. [Google Scholar]
[5]. Bradley PJ, Adenoid cystic carcinoma of the head and neck: a reviewCurr Opin Otolaryngol Head Neck Surg 2004 12(2):127-32. [Google Scholar]
[6]. Jaso J, Malhotra R, Adenoid cystic carcinomaArch Pathol Lab Med 2011 135:511-15. [Google Scholar]
[7]. Szanto PA, Luna MA, Tortoledo ME, White RA, Histologic grading of adenoid cystic carcinoma of the salivary glandsCancer 1984 54(6):1062-69. [Google Scholar]
[8]. Nomori H, Kaseda S, Kobayashi K, Ishihara T, Yanai N, Torikata C, Adenoid cystic carcinoma of the trachea and main-stem bronchus. A clinical, histopathologic, and immunohistochemical studyJ Thorac Cardiovasc Surg 1988 96:271-77. [Google Scholar]
[9]. Kansal A, Ghosh S, Tracheal adenoid cystic carcinoma mimicking bronchial asthmaIndian J Allergy Asthma Immunol 2013 27:140-42. [Google Scholar]
[10]. Calzada AP, Miller M, Lai CK, Elashoff DA, Abemayor E, St John MA, Adenoid cystic carcinoma of the airway: A 30-year review at one institutionAm J Otolaryngol 2012 33:226-31. [Google Scholar]