Primary Pure Keratinising Squamous Cell Carcinoma: A Rare Malignancy with Aggressive Behaviour
Mansi Chandna1, Leela Pant2, Malini Garg3, Garima Singh4, Sompal Singh5
1 Senior Resident, Department of Pathology, North Delhi Municipal Corporation Medical College & Hindu Rao Hospital, Delhi, India.
2 Medical Superintendent, Kasturba Hospital, New Delhi, India.
3 DNB Student, Department of Pathology, North Delhi Municipal Corporation Medical College & Hindu Rao Hospital, Delhi, India.
4 Senior Resident, Department of Pathology, North Delhi Municipal Corporation Medical College & Hindu Rao Hospital, Delhi, India.
5 Specialist, Department of Pathology, Hindu Rao Hospital, Delhi, India.
NAME, ADDRESS, E-MAIL ID OF THE CORRESPONDING AUTHOR: Dr. Mansi Chandna, Senior Resident, Department of Pathology, North Delhi Municipal Corporation Medical College & Hindu Rao Hospital, Delhi-110007, India.
E-mail: chandnamansi@gmail.com
Primary well differentiated keratinising Squamous Cell Carcinoma (SCC) is a rare gall bladder malignancy accounting for 3.3% of all gall bladder carcinomas. Here we present a case of a 70-year-old female with complaints of dyspepsia and right upper quadrant pain for 3 months. Ultrasonography showed gall stones along with thickened and irregular gall bladder wall. Grossly an exophytic growth was noted involving large part of the body of gall bladder. Histological features were of well differentiated SCC with extensive keratinisation involving full thickness of the wall. No glandular component was seen. Metastasis from other primary was ruled out after thorough work-up. SCC of gall bladder has an infiltrative growth pattern and behaves aggressively. Early diagnosis plays the most important role in increasing the survival.
Adenosquamous, Chronic cholecystitis, Gall bladder carcinoma
Case Report
A 70-year-old female presented with clinical symptoms of dyspepsia and right upper quadrant pain for 3 months. On examination, patient had mild pallor and icterus and no lymphadenopathy. Mild tenderness was present on abdominal palpation but no palpable lump was found. Ultrasonography revealed gall stones along with thickened and irregular gall bladder wall. During the open cholecystectomy procedure, gall bladder was enlarged, hard and was not adherent to any of the adjacent tissue. Liver and extra hepatic biliary tree appeared normal. No lymph nodes were detected.
Grossly the gall bladder was enlarged measuring11x5x4cm. External surface was congested. No mass was seen externally. On cutting open, gall bladder wall was irregularly thickened with an exophytic grey white tumour filling the entire body of the gall bladder measuring 7x5x4cm. A single stone was present at the fundus and the rest of the mucosa appeared atrophic [Table/Fig-1].
Cut surface of gall bladder showing grey white exophytic mass occupying the body with thickened gall bladder wall.

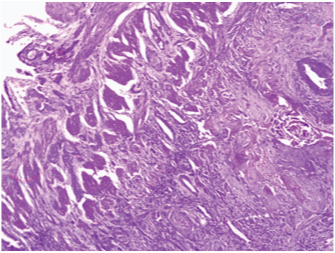
Microscopic examination revealed a well differentiated, extensively keratinising SCC, invading full thickness of the gall bladder wall and reaching up to serosa but not crossing it [Table/Fig-2,3]. Many keratin pearls and intercellular bridges were demonstrated [Table/Fig-4]. Angiolymphatic invasion was present. Atrophic mucosa showed squamous metaplasia at places [Table/Fig-5]. Resected margin of cystic duct was free of tumour. Even after extensive sampling, no evidence of glandular differentiation was seen histopathologically. Special stains for mucin also failed to demonstrate any glandular differentiation. Metastasis from any other site was excluded after extensive work up. Thus, a diagnosis of pure keratinising SCC of gall bladder was given. On follow up, no residual tumour was found.
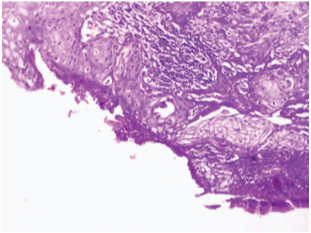
Photomicrograph showing well differentiated SCC replacing the normal glandular mucosa of gall bladder. (H&E;40X).
Photomicrograph showing islands of SCC invading the wall of gall bladder. (H&E;40X).
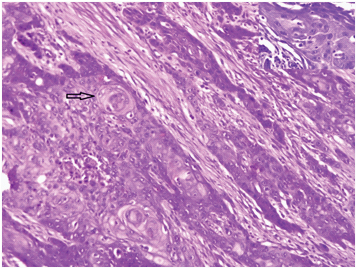
Photomicrograph showing keratohyaline pearls and dyskeratotic cells. (H&E;100X) Inset shows intercellular bridges in well differentiated SCC. (H&E; X400).
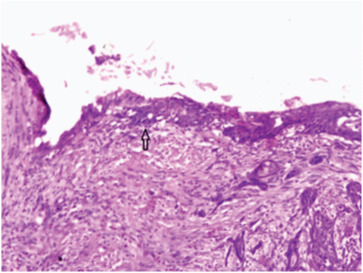
Photomicrograph showing squamous metaplasia of glandular epithelium giving rise to SCC. (H&E;40X).
Discussion
Gall bladder carcinoma is more commonly seen in older females with adenocarcinoma being the most common gall bladder malignancy [1]. SCC of the gallbladder is a rare gall bladder neoplasm accounting for about 12.7% of all cases [1–4]. It includes both adenosquamous and pure squamous cell carcinomas. Pure SCC is even less common subtype with a incidence of 0-3.3% [1–4]. Gall bladder cancer usually presents with vague symptoms which are indistinguishable from those associated with cholelithiasis: abdominal pain, jaundice, anorexia, nausea and vomiting. The majority of patients are diagnosed at an advanced, surgically unresectable, stage, and the mean 5-year survival for these patients remains less than 10% [5]. The overall prognosis of all gall bladder Carcinomas showing squamous differentiation is worse than that of adenocarcinomas.
Squamous differentiation is very rarely seen in gall bladder neoplasm and is present mostly as a secondary component in adenocarcinomas [6–8]. SCC of gallbladder is seen beyond fifth decade of life, with an obvious female predominance in the ratio of 3:1 [9,10]. Right hypochondrial pain is the most common presenting complaint occurring in 2/3rd of the patients which is followed by right hypochondrial mass and jaundice [11]. As the presentation is ill defined most patients are not suspected to have cancer, and instead undergo cholecystectomy with the diagnosis of chronic cholecystitis.
Microscopically, characteristic features of pure SCC are prominent keratinization, with abundant keratohyaline pearls, dyskeratotic cells and central deposition of dense keratin material within the infiltrative nests [1]. Present case also showed all these features.
The literature says that SCC shows trends for being locally advanced (pT2, pT3 and pT4) carcinoma and have a tendency to spread locally, especially to liver, at the time of diagnosis [6,8,12,13]. But the overall incidence of lymph node metastasis is lower than ordinary gallbladder adenocarcinoma. In the present case, tumour mass was limited to the gall bladder. The malignant cells were seen reaching up to the serosa but were not crossing it. There was no evidence of tumour in any of the adjacent tissue or lymph nodes.
SCC of the gallbladder is thought to arise from the basal cell layer of the epithelium. The aetiopathogenesis of SCC of gallbladder is difficult to determine. It is hypothesised that SCC of gall bladder arise from malignant transformation of heterotopic squamous epithelium. Others say there is malignant transformation of metaplastic squamous epithelium, while few studies propose squamous metaplasia of adenocarcinoma [9,10]. Our case also showed the presence of squamous metaplasia of the adjacent mucosa, thus favouring metaplasia-dysplasia-carcinoma sequence.
In a study of 606 gallbladder carcinomas by Roa et al., 34 cases had squamous differentiation with only 1% pure SCC. The female to male ratio of SCC was found similar to adenocarcinoma i.e., 3.8%. The mean age of presentation of SCC was 65 years with only 13% of patients suspected with carcinoma preoperatively [1]. All pure SCC had prominent keratinisation. They reported that the survival of patients with SCC/adenosquamous carcinomas was significantly worse than that of stage-matched advanced gallbladder adenocarcinoma cases [1].
Kalayarasan R et al., studied the differences between SCC and adenocarcinoma of gall bladder. They concluded that SCC present with a larger tumor size, a higher incidence of adjacent tissue involvement and less common nodal involvement [14].
Song HW et al., studied 411 gall bladder carcinomas and found that only 10 cases were of pure SCC. Mean age of presentation was 61.4 years and most common complaint was abdominal pain. Majority of the cases were pT4 stage and also had higher nodal metastases. Prognosis was poorer in SCC than the stage matched adenocarcinoma [15].
SCC of gall bladder is aggressive tumours with a poor outcome. The extent of tumour in-vasion at the time of diagnosis is the most important parameter in determining survival. Cholecystectomy is curative in T1 stage. Radical resection is the mainstay of treatment for patients with locally invasive SCC and offers the only chance for cure. Adjuvant postoperative chemotherapy and radiotherapy may be used; although their results are inconsistent and only palliative [9,10]. Early diagnosis is the most important parameter for improving the survival indices among the patients with SCC of the gallbladder.
Conclusion
Primary pure SCC of gall bladder is a rare pathological entity with vague clinical presentation. These are aggressive tumors which present at higher stage. Clinicians and pathologist should be aware of this subtype of gall bladder carcinoma to enable early detection and a better outcome in patients.
[1]. Roa JC, Tapia O, Cakir A, Squamous cell and adenosquamous carcinomas of the gallbladder: clinicopathological analysis of 34 cases identified in 606 carcinomasMod Pathol 2011 24:1069-78. [Google Scholar]
[2]. Rekik W, Ben Fadhel C, Case report: primary pure squamous cell carcinoma of the gallbladderJournal of Visceral Surgery 2011 148(2):149-51. [Google Scholar]
[3]. Soyama A, Tajima Y, Kuroki T, Radical surgery for advanced pure squamous cell carcinoma of the gallbladder: report of a caseHepatogastroenterology 2011 58(112):2118-20. [Google Scholar]
[4]. Waisberg J, Bromberg SH, Franco MIF, “Squamous cell carcinoma of the gallbladderSao Paulo Medical Journal 2001 119(1):43 [Google Scholar]
[5]. Neil D, Theise. Liver and Gallbladder. In: Vinay Kumar, Abul K. Abbas, Jon C. Aster, editorsRobbins and Cotran Pathologic Basis of Disease 2015 9th edCanadaElsevier:820-81. [Google Scholar]
[6]. Chan KM, Yu MC, Lee WC, Adenosquamous/ squamous cell carcinoma of the gallbladderJ Surg Oncol 2007 95:129-34. [Google Scholar]
[7]. Mingoli A, Brachini G, Petroni R, Squamous and adenosquamous cell carcinomas of the gallbladderJ Exp Clin Cancer Res 2005 24:143-50. [Google Scholar]
[8]. Lada PE, Taborda B, Sanchez M, Adenosquamous and squamous carcinoma of the gallbladderCir Esp 2007 81:202-06. [Google Scholar]
[9]. Karasawa T, Itoh K, Komukai M, Squamous cell carcinoma of gallbladder. Report of two cases and review of the literatureActa Pathol Jpn 1981 31:299-308. [Google Scholar]
[10]. Hanada M, Shimizu H, Takami M, Squamous cell carcinoma of the gallbladder associated with squamous metaplasia and adenocarcinoma in situ of the mucosal columnar epitheliumActa Pathol Jpn 1986 36:1879-86. [Google Scholar]
[11]. Kamat R, Pandya JS, Jashnani K, Primary squamous cell carcinoma of gallbladder presenting as empyemaBombay Hospital Journal 2001 43:447-48. [Google Scholar]
[12]. Kondo M, Dono K, Sakon M, Adenosquamous carcinoma of the gallbladderHepatogastroenterology 2002 49:1230-34. [Google Scholar]
[13]. Saito A, Noguchi Y, Doi C, A case of primary adenosquamous/squamous cell carcinoma of gallbladder directly invaded duodenumHepatogastroenterology 1999 46:204-07. [Google Scholar]
[14]. Kalayarasan R, Javed A, Sakhuja P, Squamous variant of gallbladder cancer: is it different from adenocarcinoma?Am J Surg 2013 206(3):380-85. [Google Scholar]
[15]. Song HW, Chen C, Shen HX, Squamous/adenosquamous carcinoma of the gallbladder: Analysis of 34 cases and comparison of clinicopathologic features and surgical outcomes with adenocarcinomaJ Surg Oncol 2015 112(6):677-80. [Google Scholar]