Primary Renal Lymphoma - A Case Report and Review of Literature
Shraddha Shetty1, Avinash Chandra Singh2, Vinod Babu3
1 Resident, Department of General Surgery, Victoria Hospital, Bangalore Medical College and Research Institute, Bengaluru, Karnataka, India.
2 Resident, Department of General Surgery, Victoria Hospital, Bangalore Medical College and Research Institute, Bengaluru, Karnataka, India.
3 Resident, Department of General Surgery, Victoria Hospital, Bangalore Medical College and Research Institute, Bengaluru, Karnataka, India.
NAME, ADDRESS, E-MAIL ID OF THE CORRESPONDING AUTHOR: Dr. Shraddha Shetty, Department of General Surgery, Victoria Hospital, Near City Market, Fort Road, Bengaluru-560002, Karnataka, India.
E-mail: shraddha.shetty@outlook.com
Primary Renal Lymphoma (PRL) is rare and its existence has been called into question due to the absence of lymphatic tissue within renal parenchyma. Non-specific abdominal pain with mass in the lumbar region and otherwise unexplained renal failure is the most common presentation. Almost all patients eventually develop extrarenal lymphomatous disease and few patients survive beyond one year. Surgical treatment is rarely feasible as primary modality of treatment since the tumour often encases major vessels and surrounding organs necessitating major resection. Instead, an attempt can be made to downstage the tumour with chemotherapy before attempting surgery. Here we present a case of primary renal Non-Hodgkins Lymphoma (NHL) which was treated with chemotherapy but the patient succumbed to disease before the third cycle.
B cell lymphoma, Kidney, Non-Hodgkins lymphoma, Renal tumors
Case Report
A 50-year-old male presented with history of early satiety and dull aching pain in the left loin since one month, which was progressive with no aggravating or relieving factors. On physical examination, a vague abdominal mass was palpable, predominantly occupying the left hypochondrium and lumbar regions, around 20cm x 15cm, smooth surfaced with ill defined borders and no movement on respiration, non-tender and firm in consistency. Fingers could be insinuated below the costal margin and the mass was not ballotable. There was no palpable hepatosplenomegaly. The mass was tympanitic on percussion with no bruit in the renal angle.
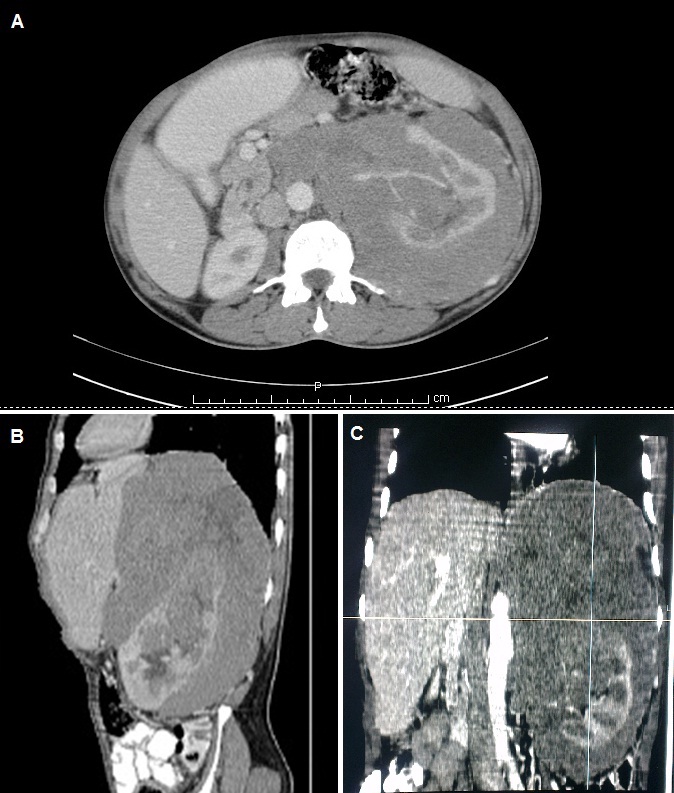
Complete haemogram showed normocytic normochromic anaemia. All other blood investigations, including renal function tests and serum electrolytes, were within normal limits. Ultrasound (USG) of the abdomen revealed a large hypoechoic mass encasing the left kidney with normal colour uptake of renal vessels. Contrast enhanced Computed Tomography (CT) of abdomen and pelvis showed a large heterogeneously enhancing hypo to isodense mass encasing the left kidney with its hilum, but normal renal artery contrast opacification. There was encasement of aorta by the mass with displacement of aorta, inferior vena cava, coeliac artery and superior mesenteric artery but no luminal compromise - suggestive of primary renal lymphoma [Table/Fig-1]. In view of extensive disease not amenable for curative surgery, neoadjuvant chemotherapy was planned.
CECT abdomen and pelvis showing (a) axial, (b) sagittal and (c) coronal view of left renal mass showing mild enhancement. Renal artery shows normal contrast opacification. There is encasement of aorta by the mass with displacement of aorta, IVC, coeliac artery and SMA but no luminal compromise.
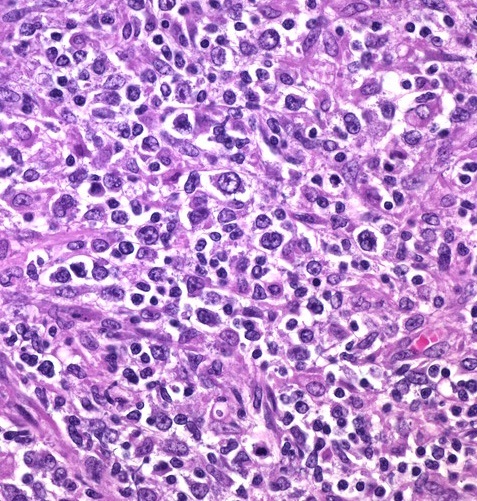
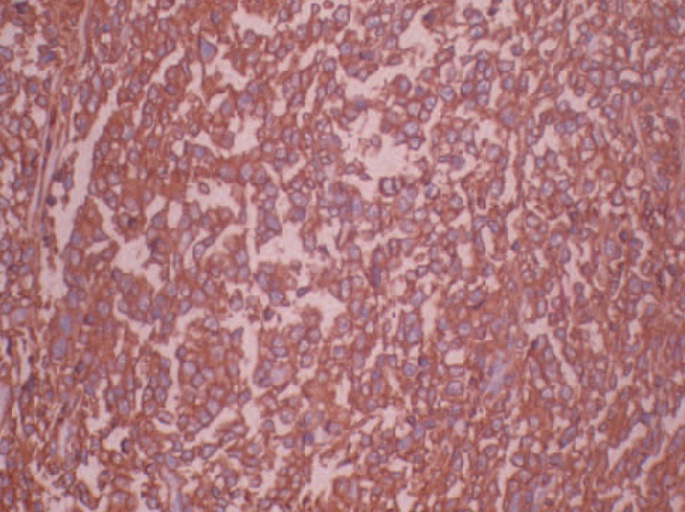
Core biopsy showed diffuse infiltration by monomorphous popu-lation of atypical lymphoid cells [Table/Fig-2]. Immunohistochemical stains were positive for bcl-2, bcl-6, CD 10 and CD 20, confirming the diagnosis of renal lymphoma [Table/Fig-3]. Bone marrow biopsy was negative. An FDG Positron emission tomography scan revealed no other focus of uptake.
Histopathological examination of core biopsy specimen showing diffuse infiltration of interstitium by monomorphous atypical lymphocytes (H&E, x400 magnification).
Immunohistochemical staining showing high CD 20 positivity. (x400 magnification).
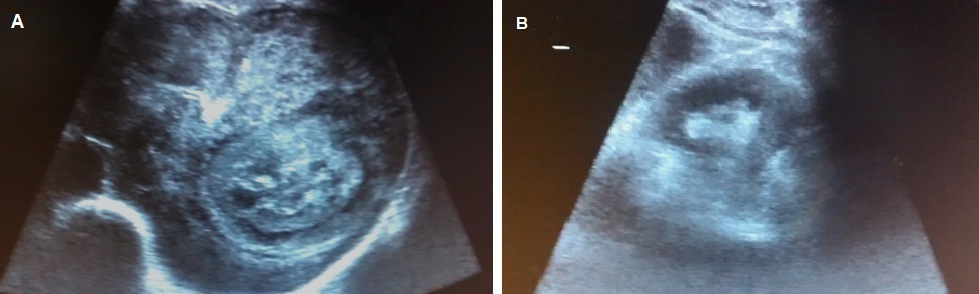
The patient was initiated on CHOP (Cyclophosphamide, Adriamycin, Vincristine) regimen. Post first cycle of chemotherapy, there was dramatic reduction in the size of the mass on USG [Table/Fig-4]. However, the patient succumbed to disease before the third cycle. Post mortem examination request was denied.
Pre-chemotherapy (a) and post-chemotherapy (b) USG appearance of renal mass.
Discussion
The usual precursor for extranodal lymphoma is specific pathologic proliferative response of lymphoid tissue at the site [1]. Primary extranodal NHL accounts for one-third of all NHL and necessarily has no involvement of nodes, spleen, Waldeyer’s ring or thymus [2], 50% NHL have renal involvement on autopsy. Less than 1% are clinically suspected [3]. Primary Renal Lymphoma (PRL) is defined as NHL arising in renal parenchyma and not invasion from an adjacent lymphomatous mass [4]. The first case was reported by Coggins in 1980 [5].
Malbrain (1994) elucidated criteria for the diagnosis of PRL:
the patient presents with acute renal failure that cannot be attributed to any other cause;
there is rapid improvement in renal function following treatment;
there is increase in the size of the kidney without urinary tract obstruction;
there is no nodal or extranodal involvement elsewhere;
diagnosis should be supported by histological findings [6].
More recently Stallone (2000), on review of 29 cases of PRL, stated that the following criteria be met for diagnosis:
there is lymphomatous renal infiltration;
there is non-obstructive uni- or bilateral renal enlargement;
there is no extra renal localisation of lymphoma at the time of diagnosis [7].
The normal kidney does not have lymphoid tissue [8]. Puente Duanay postulated that pre-existing inflammatory processes recruit lymphoid cells into renal parenchyma and while there, "untimely oncogenic event takes place" [9]. The renal capsule is rich in lymphatics and it has been hypothesized that the tumour penetrates the parenchyma from the capsule [10] or the renal sinus [11].
PRL accounts for less than 1% of all renal lesions [7]. It forms about 0.7% of the extranodal lymphomas in North America [12]. Bilateral presentation is seen in 10% to 20% of the cases [7]. Association of the tumour has been found with chronic inflammatory and infectious diseases like Sjogren’s disease, systemic lupus erythematosus, chronic pyelonephritis and Epstein Barr virus infections [7].
PRL is an infiltrative tumor that grows without disruption of structure or function [13]. However, the most common presentation of PRL is of acute renal failure, flank pain and mass. The cause for renal failure is not always straightforward but several theories have been postulated including lymphomatous infiltration of the parenchyma, hypercalcaemia due to vitamin D overproduction by the mass [14] or ureteral, vascular or tubular compression by the mass [15].
On USG, PRL appears as a hypovascular hypo to anechoic mass without posterior acoustic shadowing. Typical CT patterns of renal lymphoma include multiple renal masses (60%), renal invasion from contiguous retro peritoneal disease (25-30%), perirenal or diffuse renal infiltration (25-30%) and solitary mass (<6%) [16]. PRL should be differentiated from Renal Cell Carcinoma (RCC) in view of widely varying treatment. On CT, homogeneous post contrast attenuation points more in favour of PRL, whereas, presence of calcification, renal vein thrombosis and mass effect on renal vessels and the pelvicalyceal system is more likely to be RCC. Rarely, it may present as enlarged non enhancing kidneys, direct invasion of the renal sinus and hilum or diffuse perirenal invasion [17]. On magnetic resonance imaging, PRL exhibits lower signal intensity than normal cortex on unenhanced T1 weighted images and lower enhancement on gadolinium contrast [18]. FDG uptake is found to be higher in lymphoma than in RCC [19].
Renal biopsy has shown a sensitivity of 70% to 92% and specificity of almost 100% in the diagnosis of PRL [20]. The histological subtype most prevalent in PRL has been found to be intermediate and high grade B-cell NHL, particularly the large B-cell variant [13]. Several patterns of involvement have been described: diffuse infiltration causing marked organ enlargement, one or more discrete tumor masses or intravascular form. Intravascular large B-cell lymphoma is described as malignant lymphoid cell proliferation within lumina of blood vessels in several organs like kidneys, adrenal, skin, liver, lungs, Central Nervous System (CNS). It is a limited disease and has better prognosis [21]. Rarer still is the occurrence of renal involvement in T-cell lymphoma with five reported cases of primary T-cell renal lymphoma [15,18].
Systemic chemotherapy with or without radiotherapy has shown success in inducing remission [18] albeit short-lived. There have been isolated reports of chemotherapy inducing enough tumor regression to allow curative resection [22]. CHOP employed for treatment of NHL is the regimen of choice for PRL with inclusion of Rituximab when feasible, in view of better overall prognosis [23]. CNS recurrence has shown good response to intrathecal chemotherapy in isolated case reports [24]. PRL disseminates rapidly and upto 75% of the patients die within a year of diagnosis [13,20].
Conclusion
Despite the rarity of its existence, primary renal lymphoma should be kept in mind while investigating the differential diagnosis of a renal mass, since its treatment and prognosis varies so vastly from the usual suspects. While the diagnosis is mostly radiological, it is essential to confirm the same histologically to justify initiation of chemotherapy as the primary modality of treatment.
[1]. Freeman C, Berg JW, Cutler SJ, Occurrence and prognosis of extranodal lymphomasCancer 1972 29:252-60. [Google Scholar]
[2]. Airaghi L, Greco I, Carrabba M, Barcella M, Baldini IM, Bonara P, Unusual presentation of large B cell lymphoma: a case report and review of literatureClin and Lab Haematol 2006 28:338-42. [Google Scholar]
[3]. Risdall R, Hoppe RT, Warnke R, Non-Hodgkin’s lymphoma a study of the evolution of the disease based upon 92 autopsied casesCancer 1979 44:529-42. [Google Scholar]
[4]. Okuno SH, Hoyer JD, Ristow K, Witzig TE, Primary renal non-Hodgkin’s lymphoma - an unusual extranodal siteCancer 1995 75:2258-61. [Google Scholar]
[5]. Coggins CH, Renal failure in lymphomaKidney Int 1980 17(6):847-55. [Google Scholar]
[6]. Malbrain MLNG, Lambrecht GLY, Daelemans R, Lins RL, Hermans P, Zachee P, Acute renal failure due to bilateral lymphomatous infiltrates. Primary extranodal non Hodgkin’s lymphoma (p-EN-NHL) of the kidneys: does it really exist?Clin Nephrol 1994 42(3):163-69. [Google Scholar]
[7]. Stallone G, Infante B, Manno C, Campobasso N, Pannarale G, Schena FP, Primary renal lymphoma does exist: case report and review of the literatureJ Nephrol 2000 13:367-72. [Google Scholar]
[8]. Farrow GM, Harrison EG, Utz DC, Sarcomas and sarcomatoid and mixed malignant tumours of the kidney in adults - Part IICancer 1968 22(3):551-55. [Google Scholar]
[9]. Duanay PN, Linfosarcoma y linfosarcomatosis des los rinones: part IIRev Med. Trop Parasitol Bacteriol Clin Lab 1940 6:213 [Google Scholar]
[10]. Betta PG, Bottero G, Cosimi MF, Musante F, Primary renal lymphomaEur Urol 1986 12:352-54. [Google Scholar]
[11]. Torrecilla Garcia-Ripoll JR, Pascual Samaniego M, Martin Blanco S, Rivera Ferro J, Peral Martinez JI, Fernandez del Busto E, Primary renal lymphomaActas Urol 2003 27:555-58. [Google Scholar]
[12]. Freeman CR, Shustik C, Brisson ML, Meagher-Villemure K, Dylewski I, Primary malignant lymphoma of the central nervous systemCancer 1986 58:1106-11. [Google Scholar]
[13]. Kandel LB, McCullough DL, Harrison LH, Woodruff RD, Ahl ET Jr, Munitz HA, Primary renal lymphoma. Does it exist?Cancer 1987 60:386-91. [Google Scholar]
[14]. Levendoglu-Tugal O, Kroop S, Rozemblit GN, Weiss R, Primary renal lymphoma and hypercalcaemia in a childLeuk Lymphoma 2002 43:1141-46. [Google Scholar]
[15]. Kwakernaak AJ, Hazenberg MD, Roelofs JJTH, van Noesel CJM, van Oers MHJ, van Tellingen A, Precursor T-lymphoblastic lymphoma presenting as primary renal lymphoma with acute renal failureNDT Plus 2011 4:289-91. [Google Scholar]
[16]. El-Sharkawy MS, Siddiqui N, Aleem A, Diab AA, Renal involvement in lymphoma: prevalence and various patterns of involvement on abdominal CTInt Urol Nephrol 2007 39:929-33. [Google Scholar]
[17]. Sheth S, Ali S, Fishman E, Imaging of renal lymphoma: Patterns of disease with pathologic correlationRadiographics 2006 26:115-68. [Google Scholar]
[18]. O’Riordan E, Reeve R, Houghton JB, O’Donoghue DJ, Waldek S, Primary bilateral T-cell renal lymphoma presenting with a sudden loss of renal functionNephrol Dial Transplant 2001 16:1487-89. [Google Scholar]
[19]. Ye XH, Chen LH, Wu HB, Feng J, Zhou WL, Yang RM, 18F-FDG PET/CT evaluation of lymphoma with renal involvement: Comparison with renal carcinomaSouth Med J 2010 103:642-49. [Google Scholar]
[20]. Skarin A, Uncommon presentations of non-hodgkin’s lymphoma. case 3. primary renal lymphomaJ Clin Oncol 2003 21:564-69. [Google Scholar]
[21]. Kameoka Y, Takahashi N, Komatsuda A, Kidney limited intravascular large B cell lymphoma: a distinct variant of IVLBCL?Int J Hematol 2009 89(4):533-37. [Google Scholar]
[22]. Pingerra GM, Peschel R, Buttazzoni A, Mitterberger M, Friedrich A, Pallwein L, A possible case of primary renal lymphoma: a case reportCases J 2009 2:6233 [Google Scholar]
[23]. Vázquez-Alonso F, Puche-Sanz I, Sánchez-Ramos C, Flores-Martín J, Vicente-Prados J, Cózar-Olmo JM, Primary renal lymphoma: report of three new cases and literature reviewArch Esp Urol 2009 62:461-65. [Google Scholar]
[24]. Hu R, Zhang R, Miao M, Zhu K, Yang W, Liu Z, Central nervous system involvement of primary renal lymphoma with diffuse large B-cell type lymphomaAm J Case Rep 2013 14:292-94. [Google Scholar]