Portal Vein Thrombosis and Arterioportal Fistula in Post Liver Transplant Recipient: A Case Report
Shruti P. Gandhi1, Kajal Patel2, Vaibhav Sutariya3, Pranjal Modi4
1 Associate Professor, Department of Radiology & Imaging, G. R. Doshi and K. M. Mehta Institute of Kidney Diseases and Research Centre (IKDRC), Dr. H.L. Trivedi Institute of Transplantation Sciences (ITS), Civil Hospital Campus, Asarwa, Ahmedabad, Gujarat, India.
2 Associate Professor, Department of Radiology & Imaging, G. R. Doshi and K. M. Mehta Institute of Kidney Diseases and Research Centre (IKDRC), Dr. H.L. Trivedi Institute of Transplantation Sciences (ITS), Civil Hospital Campus, Asarwa, Ahmedabad, Gujarat, India.
3 Professor, Department of Gastrology and Tansplantation, G. R. Doshi and K. M. Mehta Institute of Kidney Diseases and Research Centre (IKDRC), Dr. H.L. Trivedi Institute of Transplantation Sciences (ITS), Civil Hospital Campus, Asarwa, Ahmedabad, Gujarat, India.
4 Professor, Department of Urology and Tansplantation, G. R. Doshi and K. M. Mehta Institute of Kidney Diseases and Research Centre (IKDRC), Dr. H.L. Trivedi Institute of Transplantation Sciences (ITS), Civil Hospital Campus, Asarwa, Ahmedabad, Gujarat, India.
NAME, ADDRESS, E-MAIL ID OF THE CORRESPONDING AUTHOR: Dr. Shruti P. Gandhi, 13-Vrundavan Apartment, Plot no 7 Laxminarayan Society, Near Santinagar Jain Temple, Usmanpura, Ahmedabad, Gujarat-380013, India.
E-mail: drshrutigandhi@gmail.com
An intrahepatic Arterioportal Fistula Refers (APF) to abnormal shunt or fistulous connection between the portal venous system and a hepatic arterial system within the liver. Here, we present a case of portal vein thrombosis with APF in post-transplant liver, developed 2 years and 6 months after transplantation. The condition was diagnosed by Triphasic CT angiography. In this case report we have discussed various causes and pathophysiology of APF with its imaging findings.
CT angiography, Haemodynamically significant, Hepatogfugal flow, Transplant liver
Case Report
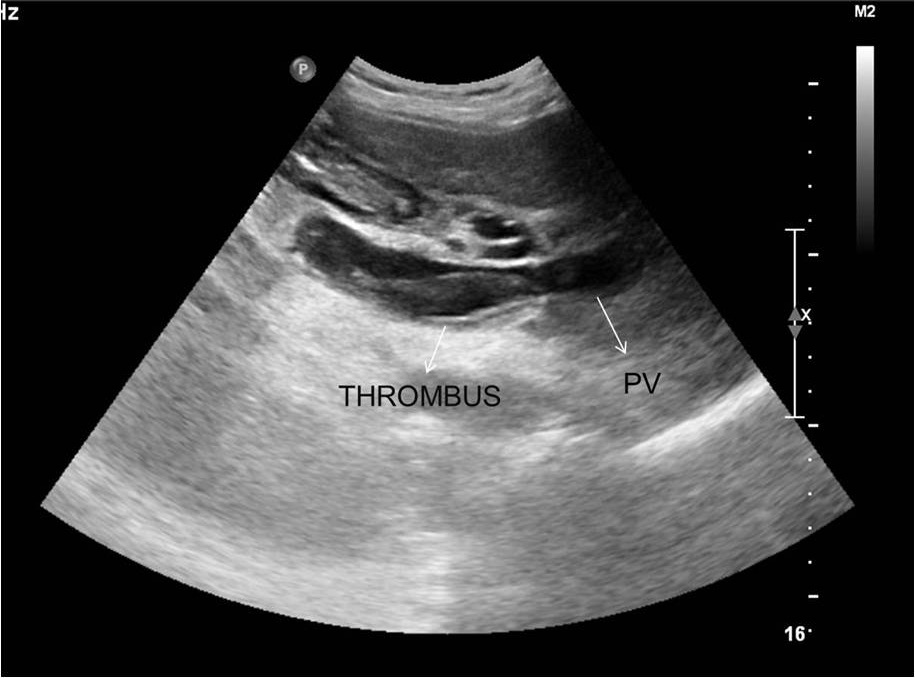
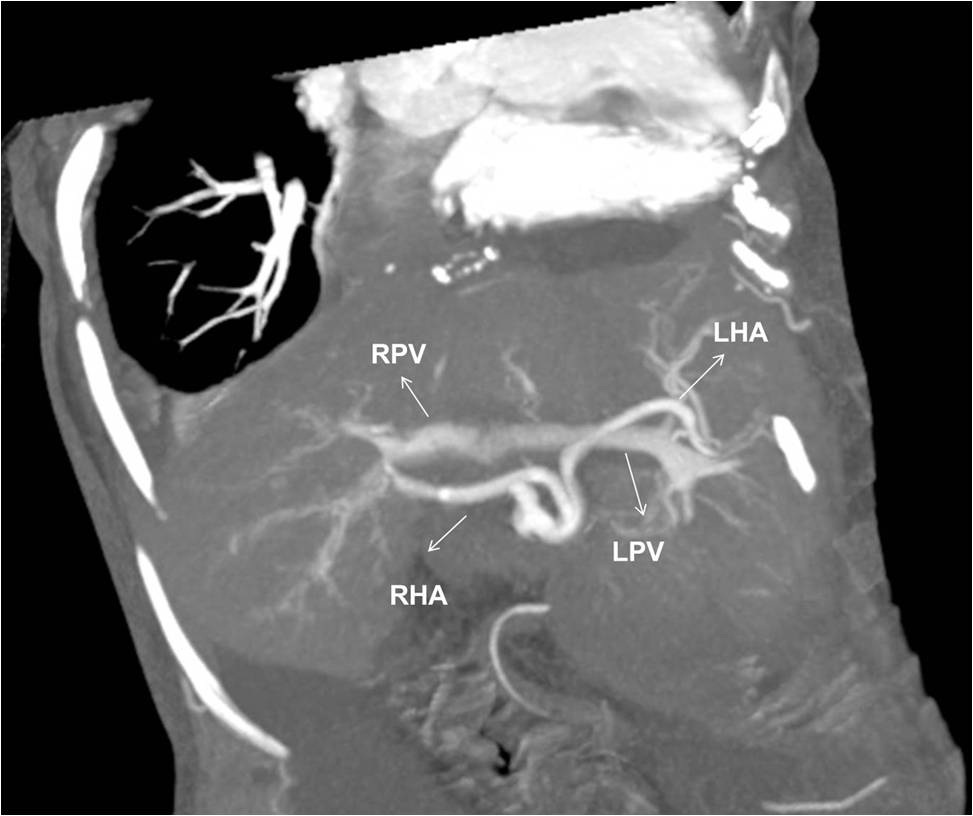
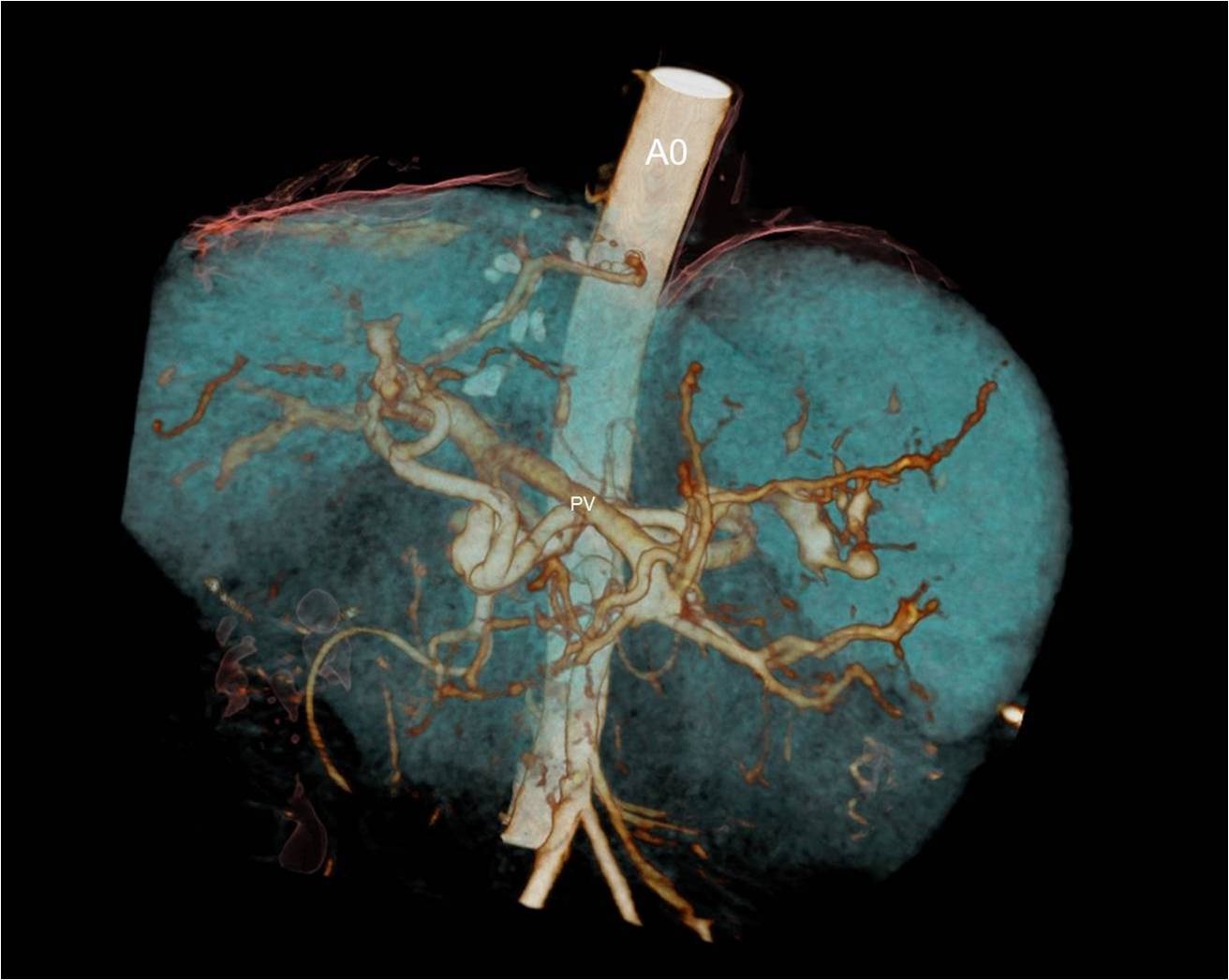
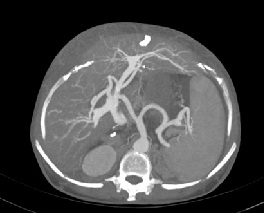
A 48-year-old female underwent deceased donor liver trans-plantation (whole graft) for HCV (genotype III) related cirrhosis. She had episode of acute cellular rejection in immediate post transplant period; for which ultrasound guided liver biopsy was done. Episode of rejection responded to steroid therapy. Rest of postoperative course was uneventful. Laboratory data at time of discharge were : Total bilirubin: 0.7 mg/dl (Direct :0.2mg/dl Indirect: 0.5mg/dl,) Total protein:6.4mg/dl, Albumin:4.1mg/dl, Globulin:2.3mg/dl, SGPT:110U/L, SGOT:100U/L, Serum alkaline phosphatase :128 U/L, Serum creatinine:0.8 mg/dl, Serum electrolytes: within normal limit. Patient was on regular follow-up and maintaining acceptable post-transplant status. One year and 11 months after transplantation, patient presented with rise in liver enzymes (SGPT: 202U/L SGOT: 320U/L) and HCV RNA quantitative (2,68,212 copies /ml). Ultrasound and doppler study of liver was normal. Patient underwent Ultrasound guided percutaneous liver biopsy (24 months after transplantation) from right anterior superior area which showed micro and macro vesicular steatosis. No evidence of changes of viral hepatitis or fibrosing cholestatic hepatitis was found. No immune injury was noted. It was reported as HCV recurrence can’t be ruled out in view of fatty changes. Patient was put on antiviral drugs but developed pancytopenia within one month after starting it. So drug was discontinued. Ultrasound and Doppler study at that time showed dilated portal vein with 36x10 mm sized partial thrombus within it [Table/Fig-1]. Hepatopetal flow was noted in rest of portal vein. Rest of Doppler findings was within normal limits. Patient was started on anticoagulants and was on regular follow-up, flow in portal vein changed from hepatopetal to hepatofugal; 4 months after detection of portal vein thrombosis. Hepatic artery resistive index was 0.52; aliasing was seen in right lobe of liver. Arterioportal fistula was suspected on Doppler study. CT angiography was advised. Triphasic CT abdomen with CT angiography was done on Somatom sensation 64 CT scan using non-ionic contrast Omnipaque 350mg/dl injected at rate of 4.5 ml/sec. CT-angiography showed contrast filling of right, left and main portal vein in arterial phase suggest multiple arterioportal communication [Table/Fig-2,3,4 and 5].
Gray scale ultrasound image showing dilated portal vein (PV) with partial thrombus within it.
CT angiography image showing contrast filling of right, left and main portal vein in arterial phase of imaging suggest multiple arterioportal communication. (RHA: right hepatic artery, LHA: left hepatic artery, RPV: right portal vein, LPV: left portal vein).
Volume rendered image of CT angiography showing multiple arterioportal fistula (Ao: aorta, PV: portal vein).
CT angiography image showing arterioportal communications between right portal vein and right hepatic artery (shown by white arrow).
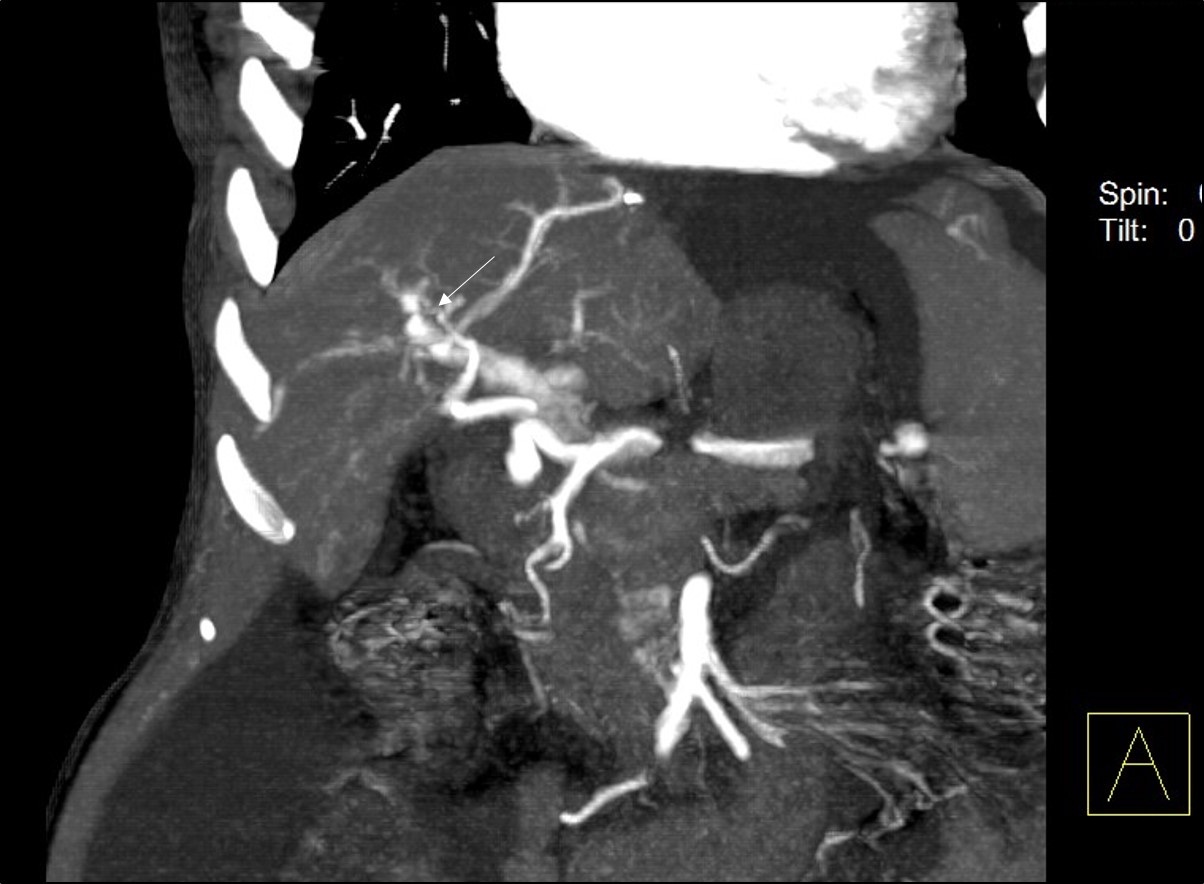
CT angiography image showing communication between left portal vein and left hepatic artery (shown by white arrow).
Discussion
Arterioportal Fistula (APF) refers to abnormal shunt or fistulous (organic or functional) connection between portal venous system and hepatic arterial system resulting in the redistribution of arterial flow into a focal region of the portal venous flow. APF can be classified as intrahepatic or extra hepatic; haemodynamically significant or haemodynamically insignificant. Haemodynamically significant arterioportal fistula were defined as those exhibiting opacification of the main portal vein of the transplanted hepatic graft or its first order branch with or without portal venous changes by Doppler ultrasound imaging. Only 0.2% cases of Haemodynamically significant APFs are reported in post liver transplant patients [1]. Incidence of APFs remain same in living related or deceased donor liver transplantation.
APF could be congenital (Hereditary haemorrhagic telangiectasia, Ehlers-Danlos syndrome and biliary atresia), idiopathic or could be secondary to cirrhosis, hepatic neoplasm, hepatic trauma, hepatic parenchymal congestion, inflammatory or infective disease, obstruction of hepatic vein or portal vein. It could be iatrogenic following percutaneous liver biopsy, cholangiogram, billiary drain placement, chemoembolization etc. Erosion of splanchnic artery aneurysms into the portal circulation is another relatively common cause of fistula and many of these occur in extra hepatic locations [2].
Most of the APFs are asymptomatic and diagnosed incidentally [3,4]. Patient may present with symptoms of portal hypertension, sepsis, haemobillia, billiary obstruction, or pulmonary hypertension [5,6]. APF tends not to produce congestive heart failure because hepatic sinusoids lying between the vascular lesion and the right heart impose a significant restriction on flow [7]. In an analysis by Saad, 73% of cases of APFs presented with abnormal liver function tests and symptoms of liver failure [3].
APF can develop via trans sinusoidal, transvasal or peribiliary route. When the hepatic vein is acutely obstructed, the pressure gradient between the sinusoids and portal veins is reversed and the portal vein becomes a drainage channel, resulting in a functional APF and increased hepatic arterial flow [8]. This route is responsible for the APF in cirrhosis or Budd-Chiari syndrome. In transvasal route, a vasa vasorum from a branch of the hepatic artery passes through the wall of a branch of the portal vein and allows blood to flow into the lumen of the portal vein due to a tumour thrombus [9]. Peribiliary vascular plexus is supplied by the hepatic artery and drains into the portal vein via interlobular veins [10]. When the portal vein is occluded, this pathway may be responsible for the most prominent arterioportal communication [11] as we assume in this case.
Doppler ultrasound is non-invasive and main screening tool for vascular complication in post-liver transplant patient. It shows turbidity and aliasing at the site of the APF. Reduced arterial Resistive Index (RI) of the feeding hepatic artery (<0.50) and >20% difference in RI or >30% difference in PI between two lobar hepatic artery should raise the suspicious of APF [12]. Reversal of flow in the receiving portal venous branch is seen. Arterialisation of flow portal vein may be seen in some cases. In one study of APF in liver transplant recipient, Doppler was able to detect only 50% of APFs; however, it detected all haemodynamically significant APF [1]. CT or MR imaging findings include early and prolonged enhancement of the peripheral portal vein, before the main portal vein is enhanced or enhancement of main portal vein before the superior mesenteric and splenic veins are enhanced. Dilated intrahepatic vessels are seen during arterial phase. CT and MR also depict the underlying cause of APF, including a tumour, inflammatory lesion and thrombosis or compression of the portal or hepatic vein. Digital subtraction angiography is the gold standard for the diagnosis.
In this case, contrast filling of main, right and left portal vein in arterial phase suggests the fistula is haemodynamically significant. Patient had history of percutaneous liver biopsy; this should raise suspicious of iatrogenic cause of fistula. But, we assume that fistula is secondary to portal vein thrombosis. Findings are in favor of: Post biopsy Hepatic artery RI was normal; Portal vein flow is reversed 4 months after development of portal vein thrombosis. Contrast filled right, left and main portal vein suggests more than one connection.
To the best of our knowledge, AP fistula secondary to portal vein thrombosis in post-liver transplant recipient is not reported in literature till date. According to literature endovascular treatment for haemodynamically significant APFs is needed in native liver rather than transplanted liver (n >280–300 versus n = 13) [13].
Relatively compromised arterial flow and the relative poor compliance of transplanted livers (stiff graft) are a hindrance to the progression of small APFs to those that are haemodynamically significant.
Some small APF can regress spontaneously. However, those shunts which are producing portal hypertension tend to persist and require treatment. Historically, first treatment performed was surgical ligation of artery supplying fistula or simple resection of vascular anomaly. Now-a-days percutaenous embolization of APF is treatment of choice. As there are intrahepatic collateral circulation pathways, the embolization of all hepatic artery branches communicating with portal branches from distal to proximal part of fistula can be necessary. Several embolization sessions may be needed. Potential complication is hepatic infarction. Post embolization follow-up should be primarily performed by Doppler ultrasound to evaluate for the reversal of signs of haemodynamic significance. In some cases re-liver transplantation represents last therapeutic resource. In our case, opinion of interventional radiologist and cardiologist was taken; due to multiple arterioportal communications and portal vein thrombosis embolization of hepatic artery was not feasible; so patient is a candidate for re liver transplantation.
Conclusion
APF following portal vein thrombosis in post transplant liver is rare. Hepatofugal portal venous flow reflects various pathologic conditions after liver transplantation and demands detailed evaluation. MDCT with CT angiography is the sensitive and non invasive modality for diagnosis of APF.
[1]. Saad WE, Davies MG, Rubens DJ, Endoluminal management of arterioportal fistulae in liver transplant recipients: a single-center experienceVasc Endovascular Surg 2006 40(6):451-59. [Google Scholar]
[2]. Vanway CW, Crane JM, Riddell DH, Foster JH, Arteriovenous fistula in the portal circulationSurgery 1971 70:876-90. [Google Scholar]
[3]. Saad WE, Lippert AJ, Davies MG, Prevalence, presentation, and endovascular management of haemodynamically or clinically significant arterio-portal fistulae in living-and cadaveric-donor liver transplant recipientsClin Transplant 2012 26(4):532-38. [Google Scholar]
[4]. Jabbour N, Reyes J, Zajko A, Arterioportal fistula following liver biopsy. Three cases occurring in liver transplant recipientsDig Dis Sci 1995 40(5):1041-44. [Google Scholar]
[5]. Figueras J, Soubrane O, Pariente D, Devictor D, Houssin D, Fatal haemobilia after liver graft biopsy in a transplanted childGastroenterol Clin Biol 1994 18(8–9):786-88. [Google Scholar]
[6]. Chua DY, Pavillion G, Tay KH, Tan JL, Chung AY, Pulmonary hypertension: an unusual presentation of an iatrogenic hepatic arterioportal fistula and its successful resolution post-embolotherapyAm Surg 2009 75(6):511-14. [Google Scholar]
[7]. Foley WJ, Turcotte JG, Hoskins PA, Brant RL, Ause RG, Intrahepatic arteriovenous fistulas between the hepatic artery and portal veinAnnals of Surgery 1971 174(5):849-55. [Google Scholar]
[8]. McCuskey RS, A dynamic and static study of hepatic arterioles and hepatic sphinctersAm J Anat 1966 119:455-77. [Google Scholar]
[9]. Bookstein JJ, Cho KJ, Davis GB, Dail D, Arterioportal communications: observations and hypotheses concerning transsinusoidal and transvasal typesRadiology 1982 142:581-90. [Google Scholar]
[10]. Cho KJ, Lunderquist A, The peribiliary vascular plexus: the microvascular architecture of the bile duct in the rabbit and in clinical casesRadiology 1983 147:357-64. [Google Scholar]
[11]. Itai Y, Matsui O, Blood flow and liver imagingRadiology 1997 202:306-14. [Google Scholar]
[12]. Bolognesi M, Sacerdoti D, Bombonato G, Chiesura-Corona M, Merkel C, Gatta A, Arterioportal fistulas in patients with liver cirrhosis: usefulness of color Doppler US for screeningRadiology 2000 216(3):738-43. [Google Scholar]
[13]. Saad WEA, Arterio portal fistuals in liver transplant recipientsSemin intervent. Radiol 2012 29(2):105-10. [Google Scholar]