Periodontal disease is one of the most common afflictions of man. It is an inflammatory condition of the supporting tissues of the teeth that results in progressive destruction of the tooth attachment and its surrounding bone [1]. Regeneration is defined as the reproduction or reconstitution of a lost or injured part [2]. Regeneration aims to restore the structure and function of the periodontium. It stimulates a cascade of coordinated healing events resulting in completion of integrated tissue formation [3]. Studies have documented the effects of bone grafts and their substitutes on the outcome of periodontal regenerative procedures. They are mainly used in an intra-bony or furcation defect. These grafts manipulate the biological response into a regenerative periodontal healing [4]. Alloplasts are one of the most frequently used grafts. An alloplast is a synthetic graft or inert foreign body implanted into tissue [5]. There has been a constant research on the particle size of the graft material which has led to the emergence of a novel product that is NHA paste. Nanocrystalline Hydroxyapatite paste (NHA paste) is a newly developed, fully synthetic and fully resorbable, injectable nanocrystalline paste [Ca10(PO4)6(OH)2] and consists of a suspension of pure Hydroxyapatite (HA) A in water. It has needle shaped crystals as shown in electron microscope. Basically, like other bone substitutes it also acts as an osteoconductive material. However, the NHA bone graft offers various advantages like its close contact with the surroundings and a high number of molecules on its surface. It also offers the benefit of undisturbed osseo-intergration and resorption over a period of 12weeks [6].
The aim of the present study was to evaluate clinically and radiographically the comparative efficacy of NHA paste and DBM as bone graft materials in the treatment of human periodontal intra-osseous defects. To clinically evaluate reduction in Probing Pocket Depth (PPD), gain in Clinical Attachment Level (CAL) and change in Gingival Margin (GM) position using DBM and NHA paste in the intra-bony defects. To radiographically evaluate, osseous defect fill using Demineralized Bone Matrix (DBM) and NHA paste in the intra-bony defects.
Materials and Methods
A total of 15 patients were assessed for the present randomized trial, from the Outpatient Department of Periodontology and Implantology, M.S. Ramaiah Dental College and Hospital, Bengaluru, Karnataka, India. It was a randomized controlled clinical trial study. Total duration of the study was 1 year which included follow-up of the patients. Out of the total 15 patients, 3 patients did not meet inclusion criteria and out of 2 patients one did not turn up and one refused to participate. Therefore, total 10 patients (7 Males and 3 Females) with 26 sites in the age group of 20-45years were selected after completion of Phase I therapy. These selected sites were divided into control and experimental sites randomly by lottery method and were treated according to split mouth technique shown in: [Table /Fig-1].
CONSORT Flow chart of the study.
Experimental sites: Open flap debridement followed by placement of NHA paste.
Control sites: Open flap debridement followed by placement of DBM.
Patients with age group between 25-50 years were selected for the study with Probing Depth (PD) ≥5mm and clinical attachment loss ≥5mm, who were systemically healthy, had not undergone any type of regenerative periodontal therapy six months prior to the initial examination. There was radiographic evidence of periodontal osseous defects and those patients were excluded who were medically compromised or undergoing therapeutic regimen that decreased the probability of soft tissue and bone healing. Pregnant or lactating patients and the patients who were allergic to materials and drugs used or prescribed in this study and smokers were excluded.
Study Design: The following clinical parameters were recorded at baseline 3, 6, 9 and 12 months post operatively which included vertical measurements for determination of PD, CAL and GM position which included distance from the Fixed Reference Point (FRP) to the Base Of Pocket (BOP), Fixed Reference Point (FRP) to Cementoenamel Junction (CEJ), Fixed Reference Point (FRP) to GM.
Customized acrylic stents with grooves were prepared on the study model of the patients. The recordings were made using a PCP-UNC 15 probe (Hu-Friedy’s). Occlusal stents for vertical probing was made [Table/Fig-2]. Probe penetration was done in the same plane with the help of grooves which helped further for recording the measurements. The lower limit of the vertical grooves was kept as reference point (Samual E. Lynch, 1992) [7].
Stent with Pcp Unc-15 probe in place.
Distance from FRP to CEJ was measured at the time of surgery in cases having no gingival recession. The clinical measurements recorded included Probing depth; (FRP to BOP –FRP to GM), CAL; (FRP to BOP – FRP to CEJ), Gingival Margin Position; (FRP to CEJ – FRP to GM).
Radiographic Measurements: Intra-oral periapical radiograph and digital radiograph (RVG) of each defect site were exposed at baseline, 3, 6, 9 and 12 months post-surgery using long cone/paralleling technique.
Bone Graft Used in the Study: DBM (Osseograft) [Table/Fig-3a]: It is a xenogenic graft material and the advantages and disadvantages of using xenografts are similar to those of using allografts and when the graft is DBM it combines all the necessary conductive features of a carrier, serving at the same time as a natural source of inductive osteo- and chondrogenic factors such as Bone Morphogenetic Proteins (BMPs). In addition, DBM is slowly biodegradable and non-immunogenic.
Demineralized bone matrix bone graft.
Nanocrystalline hydroxyapatite paste (Nanostim) [Table/Fig-3b]: A novel variant of HA alloplast is NHA paste used in augmentation of osseous defects. NHA paste is an easily flowable paste, with osteoconductive properties and is bioresorbable. In comparison to other materials it is not sintered with a very high specific surface and, the small particle size facilitates further resorption.
Nanocrystalline hydroxyapatite paste.
After initial examination and treatment planning, Phase I peri-odontal therapy was done and patient were explained about self performed plaque control measures. Selective grinding in the cases with traumatic occlusion was considered. Four to six weeks after Phase I therapy the patients were subjected to surgical procedure.
Surgical Procedure [Table/Fig-4,5,6,7,8,9,10,11 and 12]: The patient was made to rinse with 0.2% Chlorhexidine di-gluconate mouthrinse for 30 seconds prior to the surgery. Local anesthesia was obtained using 2% lignocaine with adrenaline 1:80,000 using block and infiltration techniques. A full thickness mucoperiosteal flap was raised following crevicular incision on both facial and lingual side, to provide access to the defect. Granulation tissue was removed and the root surface was planed [Table/Fig-4,5]. The surgical area was thoroughly debrided and irrigated to get a clear intra-bony defect. In the experimental sites defects were filled with NHA paste. The required quantity of NHA paste was transferred from the syringe to the dappen dish and it was delivered in to the vertical defect in small increments. Carefully each defect was packed down adequately till the level of the remaining bony walls of the defect. Control sites were grafted in a similar manner with DBM graft material. Flaps were then repositioned and secured in place using 3-0 black braided silk and interrupted sutures were placed to obtain primary closure. The surgical areas were protected with a non-eugenol dressing (Coe-pack). Combination of systemic doxyclycine HCL 200mg (loading dose) for the first day followed by 100mg/day for another four days, Ibuprofen 400mg and Paracetamol 325mg given twice daily for three days. Post-operative instructions were given. Patients were instructed for follow-up after 24 hours and then after one week.
Surgical procedure (Experimental Site) crevicular incision.
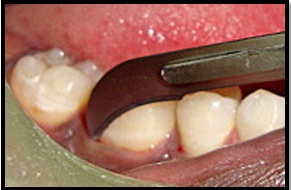
Full thickness flap reflection.
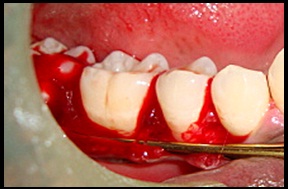
Debridement of defect done.
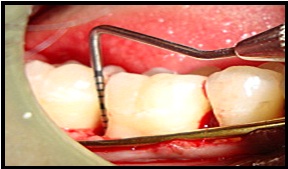
Placement of nanocrystalline hydroxyapatite paste.
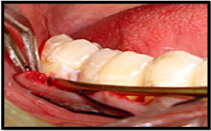
Sutured removed after 1 week (post-operatively).
Surgical procedure (control), full thickness flap reflection.
Debridement of defect done.
Placement of demineralized bone matrix.
Sutures removed after one week (Post-operatively).
At seven days following surgeries, the dressing and sutures were removed. The patients were asked regarding discomfort, swelling, pain and sensitivity. Any sign of swelling, infection, hematoma or necrosis was noted and if needed the dressing was again replaced for another one week.
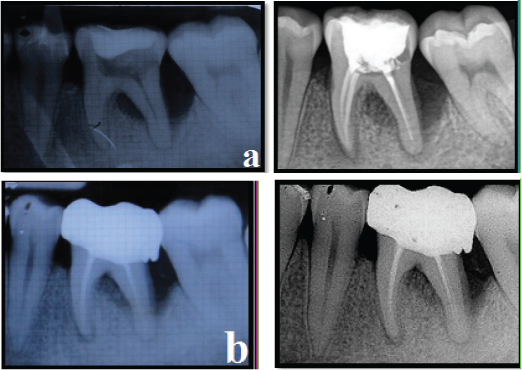
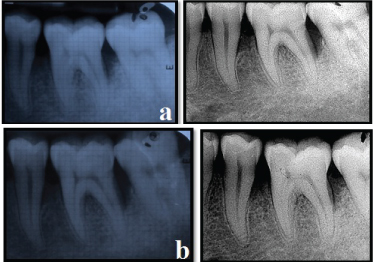
The amount of bone fill was assessed on conventional IOPA radiographs and RVG postoperatively using the AUTOCAD 2010 software [Table/Fig-13,14].
Control site radiographic assessment - IOPA and RVG
(a) Control site IOPA and RVG (Pre-operative)
(b) Control site IOPA and RVG (Post-operative 12 months)
Experimental site radiographic assessment - IOPA and RVG
(a) Experimental site IOPA and RVG (Pre-operative)
(b) Experimental site IOPA and RVG (Post-operative 12 months)
The following methods of statistical analysis have been used in this study which included Student “t” test to determine statistical difference between experimental and control groups and One way analyses of variance (ANOVA) to test the difference between groups. The Excel and SPSS (SPSS Inc, Chicago) software packages were used for data entry and analysis.
Results
The mean of percentage of bone fill in the control group obtained was 46.84%, where as the percentage of bone fill in experiment group was 48.31% (p<0.05).
The results of the clinical parameters recorded as follows: Changes in PD measurements [Table/Fig-15]: There were significant changes noted from baseline to 3,6,9,12 months in both groups in terms of all measurements. The experimental group showed highly significant reduction in PPD from baseline to 12 months. However, no major difference was noted in probing pocket depth at 3,6,9,12 months in the experimental group. Similar changes were noted in the control group showing significant decrease in PPD from baseline to 12 months. Further evaluation revealed that the difference between the experimental and control groups with respect to these measurements of PPD were statistically non-significant at all the visits.
Comparison of mean pocket depth measurements between experimental and control group at different visits.
| Time Interval | Mean Pocket Depth± SD | p-value |
|---|
| Control (n1=12) | Experimental Group (n2=12) |
|---|
| Baseline | 7.92±1.621 | 6.83±1.030 | 0.064 |
| 3 months | 4.58±0.669 | 4.17±0.577 | 0.116 |
| 6 months | 3.67±0.888 | 3.33±0.651 | 0.306 |
| 9 months | 2.75±0.622 | 2.83±1.115 | 0.823 |
| 12 months | 2.33±0.778 | 2.17±0.771 | 0.591 |
*Student “t” test, p < 0.05 Significant
Changes in CAL measurement [Table/Fig-16]: The mean CAL (±Standard Deviation) in mm was 6.92±1.311 at baseline and 2.58±1.165 at 12 months. The changes in clinical attachment level when compared to baseline were highly significant at 3,6,9 and 12 months post-operatively. The mean CAL (±Standard Deviation) in mm in control group was 7.58±1.730 at baseline and 2.92 ±1.165 at 12 months. The changes in CAL when compared to baseline were highly significant at 3,6,9 and 12 months post-operatively similar to the experimental group. However, between the groups the mean CAL measurements did not differ between the experimental and control groups at baseline 3,6,9 and 12 months showing that the difference between the groups at all visits was non-significant.
Comparison of changes in mean clinical attachment level measurements in experimental and control group at different visits.
| Time Interval | Mean Clinical Attachment Levels ± SD | p-value |
|---|
| Control (n1=12) | Experimental Group (n2=12) |
|---|
| Baseline | 7.58±1.730 | 6.92±1.311 | <0.001 |
| 3 months | 4.92±1.165 | 4.33±1.155 |
| 6 months | 4.00±1.128 | 3.83±1.030 |
| 9 months | 3.25±1.215 | 3.33±1.073 |
| 12 months | 2.92±1.165 | 2.58±1.165 |
ANOVA, p<0.05 significant
Changes in GM position [Table/Fig-17]: The mean gingival margin level (±Standard Deviation) in mm were 0.00±1.414 at baseline -0.17 ± 1.33 at 3 months, -0.50 ± 1.44 at 6 months,-0.50 ± 1.44 at 9 months and -0.50 ± 1.44 at 12 months respectively in the experimental group. The difference between the means of baseline and 12 months was found to be statistically significant but there was no significant difference between 3rd, 6th, 9th and 12th visit in the experimental group regarding gingival margin. The mean gingival margin level (±Standard Deviation) in mm was 0.33 ± 1.231 at baseline, -0.17±1.403 at 3 months, -0.33±1.497 at 6 months, -0.33±1.435 at 9 months and 0.33±1.435 at12 months and results similar to experimental group were noted between the visits. Further between the groups there was no significant difference noted with regards to gingival margin position at all the visit intervals.
Comparison of mean gingival margin position measurements between experimental and control group at different visits.
| Time Interval | Mean Gingival Margin Position ± SD | p-value |
|---|
| Control (n1=12) | Experimental Group (n2=12) |
|---|
| Baseline | 0.33±1.231 | 0.00±1.414 | <0.001 |
| 3 months | -0.17±1.403 | -0.17±1.337 |
| 6 months | -0.33±1.497 | -0.50±1.446 |
| 9 months | -0.33±1.435 | -0.50±1.446 |
| 12 months | -0.33±1.435 | -0.50±1.446 |
*Student “t” test, p < 0.05 Significant
Changes in the digital images (RVG) bone level measurements [Table/Fig-18]: The mean radiographic bone level (±Standard Deviation) in mm was 5.83±1.856 at baseline and 2.983±1.485 at 12th month in the experimental group and 5.925 ± 1.888 at baseline and 3.00 ±1.2774 at the 12th month in the control group. There was no statistical significant difference in the radiographic bone level between the groups when the baseline and 12th month measurements were compared. The mean percentage of bone fill in the control group obtained was 48.16% where as the percentage of bone fill obtained in the experimental group was 48.64%. The percentage of bone fill calculated were compared between experimental and control group, showed no statistically significant difference in the radiographic bone level, although there was statistically significant gain in bone level in both groups from baseline to 12th month.
Changes in the RVG bone level measurements at baseline and 12 months in experimental group and control group.
| Time Interval | Mean Gingival Margin Position ± SD | p-value |
|---|
| Control (n1=12) | Experimental Group (n2=12) |
|---|
| Baseline | 5.925±1.8888 | 5.833±1.8568 | <0.001 |
| 12 months | 3.008±1.2774 | 2.983±1.4856 |
Student “t” test, p>0.05 Non significant
Discussion
Intra-bony defects represent a major challenge for the clinician. These defects often require access flap surgery alone or in association with bone-regenerative techniques. Periodontal regeneration is defined as re-establishment of the lost supporting tissues including alveolar bone, cementum and periodontal ligament. For introducing a regeneration of the periodontal tissues, there have been various modalities that have been introduced in the periodontal surgery. One such treatment strategy is the use of bone grafts [8].
The function of bone grafting material is to act as structural scaffold. A wide variety of bone grafts are available which include bone autografts, bone allografts, xenografts as well as bone graft substitutes. It has been found that all bone grafting materials do not support the formation of new periodontal apparatus, however, there is histological evidence which supports the fact that periodontal regeneration can be achieved with bone replacement grafts in human beings [9].
Regenerative periodontal therapy attempts to restore lost peri-odontal structures and functional attachment through the regeneration of cementum, periodontal ligament, and alveolar bone [10].
Reduced PD, increased CAL and radiographic evidence of bone formation can be considered as the clinical outcome parameters of a successful regenerative therapy. Histologic evaluation of the defects showed evidence of periodontal regeneration [11]. Bovine-derived HA bone replacement grafts increase the available surface area that can act as an osteoconductive scaffold due to their porosity and have a mineral content comparable to that of human bone, allowing them to integrate with host bone. They have been used with success for the treatment of intra-bony defects and ridge augmentation. This process of bone growth associated with demineralized bone matrix is called endochondral ossification in which cartilage forms prior to bone [12].
Nanocrystalline Hydroxyapatite Paste (Alloplast): An alloplast is a commercially available synthetic graft implanted into tissue. Various types of alloplastic materials are commercially available which include nonporous HA, HA cement, porous HA (replamineform), beta tricalcium phosphate, PMMA and HEMA polymer (a calcium defects layered polymer of polymethylmethacrylate and hydroxyethylmethacrylate), and bioactive glass.
Sintered HA bone substitutes have been found unable to undergo sufficient physiological remodeling. This problem can be attributed mainly to sintering process, in which the mineral is heated beyond 1000oC. This leads to the transformation of the tiny (<60nm) primary HA crystallites into significant larger crystals. These larger crystals become difficult for osteoclast to break down, thus causing poor bioresorbability and limited handling characterstics [13].
Hence, a new technology has been used for the production of NHA paste to overcome the disadvantages of other alloplasts. It is manufactured by a wet chemical reaction using CaO dispersed in water under constant stirring to maintain a suspension state and H3PO4 as starting material under a constant ph. The CaO and H3PO4 suspension containing 5.5% nanosized HA crystals, with a pH of 7.5 is now concentrated using filtration and subsequent evaporation process. This leads to the formation of a highly viscous paste containing 35% of HA which enables it to act as a haemostatic agent and helps to be applied in close proximity with bone having a tight seal. The NHA paste is biocompatible, osteoconductive, non-toxic, non-inflammatory, and non-immunogenic alloplast [14].
Strietzel et al., made use of nanocrystalline HA paste in lateral ridge defects. On histological assessment after 7 months it was found that there was bone gain in those sites. Also, highlighted that resorption of NHA paste takes place over 6-7 months expressing its viability as an effective bone graft [15]. The grafts cellular effects have also been assessed and Heinz B et al., reported that NHA paste has a promising influence on proliferation of periodontal ligament cells which form the key cell population in bone regeneration [16]. This safe and osteoconductive NHA paste appears suitable for filling bone defects and bone cavities, showing resorption and a rapid osseous integration. Clinical results are comparable to those obtained in patients treated with autografts, and histology reveals that there is uneventful healing and active resorption of the alloplast, performed by osteoclasts.
Demineralized bone matrix (Osseograft): One of the recent advancements of the graft’s ability to cause bone regeneration included a study done by Tietmann et al., where 241 intra-bony defects in human dentition were studied and it was found that there was significant bone regeneration with the graft materials [17].
Erika, from his investigation showed that DBM provided one of the best materials for sinus augmentation which had an equivalent regeneration like autogenous bone graft [18]. The above findings were further complimented by Stephen, who in his SEM findings said that the DBM was as effectual as autogenous bone graft hence, placing the graft in the genre of most ideal regenerative products [19]. DBM has been tested at molecular, cellular and radiographic level in different studies and it has been proven that the grafting material holds a promising outcome in varied applications. The current study is also an add-on when it came to the clinical and radiographic findings in the intra-bony defects after the grafting of DBM.
Our study showed reduction in PPD at all the sites in the control group which was a mean reduction of 2.25mm from baseline to 12th month, this finding was similar to the results achieved by other authors. Previously Wiesen and Mellonig, had found statistically significant improvement with relation to PPD on usage of DBM [20,21]. Kaya et al., reported that DBM in any available form causes significant reduction in probing depths in the intra-bony defects [22]. A similar outcome was observed by Sara A, where she found a 3.6mm reduction in probing depths over a period of 6 months on surgical re-entry [23]. Our study also followed the findings mentioned in the above stated studies when the probing depth in control group was recorded over a 12-month period. However, the probing depth reduction in the experimental group was also statistically significantly over a period of 12 months and it was similar to that observed by Heinz et al., where reduction in mean PD from 8.3 ± 1.2 to 4.0 ± 1.1 mm was seen [16]. Chitsazi M et al., in 2011, on comparing NHA paste with autogenous bone graft found that there was significant reduction in PD in the NHA paste group [24].
When 12 defects each in test and control group were subjected to NHA paste and DBM respectively, change in PD observed was 67.45% in test group and 69.03% in control group at 12 months follow – up which was in agreement with Schwarz et al., who compared the two products in peri-implantitis defects in combination with a membrane [25]. They concluded that NHA paste was an equally effective graft for periodontal regeneration as DBM which was consistent with our findings.
Limitation
The study was limited to a relatively small sample size. More such studies with large number of subjects need to be conducted to confirm the clinical outcomes and studies that involve evaluation of the bone regeneration achieved with histomorphometric analyses of new bone formed can be planned. If found successful, NHA paste along with DBM can serve as the next generation of bone replacement materials for periodontal regeneration. Besides these, NHA paste needs to be studied with different combination therapies to establish its usage in periodontal therapy.
Conclusion
Therefore, within the limits of the current study, it can be concluded that application of both NHA paste and DBM can lead to significant improvements of the investigated clinical and radiographic parameters. However, further long term controlled studies are needed for evaluating the adjunctive benefits of NHA paste in the treatment of human periodontal osseous defects.
*Student “t” test, p < 0.05 Significant
ANOVA, p<0.05 significant
*Student “t” test, p < 0.05 Significant
Student “t” test, p>0.05 Non significant