Pure Large Cell Neuroendocrine Carcinoma of the Ovary with Metastasis to Cervix: A Rare Case Report and Review of Literature
Lakshmi Agarwal1, Bhawna Gupta2, Ayushi Jain3
1 Assistant Professor, Department of Pathology, Govt Medical College, Kota, Rajasthan, India.
2 Senior Resident, Department of Pathology, Govt Medical College, Kota, Rajasthan, India.
3 Senior Resident, Department of Pathology, Govt Medical College, Kota, Rajasthan, India.
NAME, ADDRESS, E-MAIL ID OF THE CORRESPONDING AUTHOR: Dr. Lakshmi Agarwal, Assistant Professor, Department of Pathology, Govt Medical College Kota-324010, Rajasthan, India.
E-mail: drlaxmiagarwal@gmail.com
Ovarian Large Cell Neuroendocrine Carcinoma (LCNEC) is a recently described rare entity, which even more rarely occurs in a ‘pure’ form without any associated surface epithelial-stromal or germ cell component. Cervix metastasis of ovarian LCNEC has not been reported previously. We report here a case of ovarian LCNEC in a 35-year-old female who presented with abdominal pain and amenorrhea. Grossly the left ovary showed a solid cystic tumour measuring 6 cm in diameter. Histological examination showed a pure LCNEC without any associated component, confirmed by immunohistochemistry. Metastatic tumour deposits with numerous lymphovascular emboli were identified in the cervix. A comprehensive review of literature along with the various differential diagnosis is discussed.
Cervical metastasis, Poor prognosis, Pure
Case Report
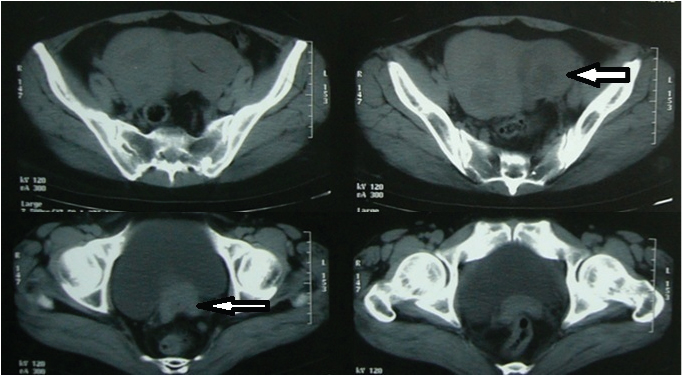
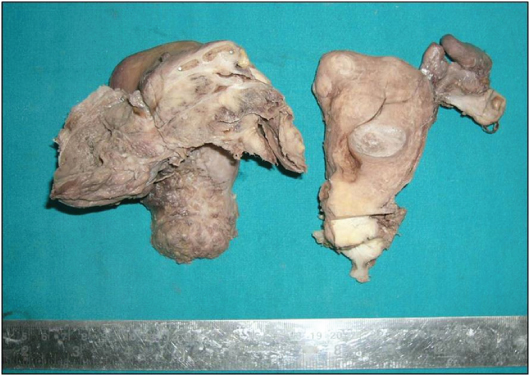
A 35-year-old married female presented with complaints of pain in abdomen and amenorrhea since 2 months and per vaginal bleeding since 1 day. On examination a hard mass was felt in the left fornix. Investigation revealed a heterogeneously enhancing soft tissue mass with cystic areas and internal septation along left adnexa and multiple uterine intramural fibroids [Table/Fig-1]. Multiple perirectal, obturator, preaortic, aortocaval and mesenteric lymph nodes were enlarged. Both CA-125 and Carcinoembryonic Antigen (CEA) were raised, while Alpha Feto Protein (AFP) and Human Chorionic Gonadotropin (HCG) were normal. Peritoneal washings were negative for malignant cells. On gross examination [Table/Fig-2], the left adnexal mass was solid cystic, grey white to brownish black and friable with areas of necrosis measuring 6x6x5 cm. The right ovary was unremarkable. The uterus showed multiple intramural fibroids varying in size from 0.3-2 cm. The cervix appeared hypertrophied and showed an ill defined fibrotic thickening.
CECT scan showing enhanced adnexal mass with central necrosis (thick arrow), and cervical thickening (thin arrow).
Gross photograph of the hysterectomy with bilateral salphingo-oophorectomy specimen showing left sided solid cystic ovarian tumour measuring 6x6x5 cm. The cut surface is grey-white to tan brown and fleshy with intact capsule. The tumour is not adherent to the uterine surface. The cervix shows an ill-defined grey-white trabeculated area extending till the isthmus.
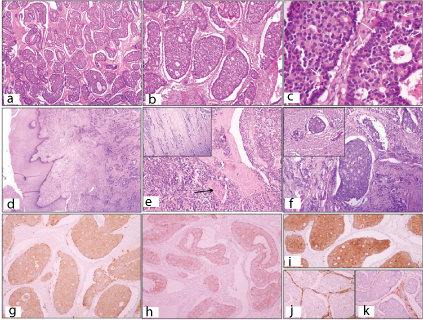
Microscopic examination of the adnexal mass showed tumour cells arranged in an insular pattern in majority of the areas. The nests were surrounded by scant ovarian stroma. Inside the tumour islands, the cells were arranged in a cribriform or follicular pattern around central clear space, and showed peripheral palisading [Table/Fig-3a,b]. The tumour cells showed moderate amount of granular eosinophilic cytoplasm with intermediate sized, round to oval nuclei with a coarse chromatin giving a stippled appearance. Mitosis was brisk, 4-5 per High Power Field (HPF) [Table/Fig-3c]. Sections from the cervix also showed the same tumour, seated in the cervical wall deep to the epithelium, extending till the muscle wall of the isthmus. The overlying cervical epithelium was predominantly intact with focal ulceration [Table/Fig-3d,e]. Numerous lymphovascular emboli were seen [Table/Fig-3f]. There was no contiguous spread of the tumour from the ovary to the uterus. Perineural invasion was also identified. Sections from the body of uterus showed leiomyomas with adenomyosis. Immunohistochemistry revealed, the tumour cells were immunopositive for synaptophysin, chromogranin and cytokeratin and immunonegative for inhibin, vimentin and CD99 (MIC-2) [Table/Fig-3g-k]. With these features a diagnosis of large cell neuroendocrine carcinoma of the ovary metastatic to cervix was offered.
Tumour cells arranged in nests and few trabeculae (H&E, x 40); b) Peripheral palisading and arrangement of tumour cells simulating a Call-Exner body (H&E, x 100); c) Tumour cells arranged in rosette-like appearance with numerous mitotic figures (white arrows) (H&E, x 400); d) Cervix showing intact ectocervical epithelium with no dysplasia and underlying infiltrating tumour cells (H&E, x40); e) Perineural invasion (black arrow), (H&E, x100); f) Lymphovascular evasion in cervix, (H&E, x 100); g) IHC- Positivity for synaptophysin; h)chromogranin; i)CK; and j,k) Negativity for Vimentin and inhibin.
Discussion
The LCNEC of ovary is an aggressive tumour. Since its original description by Collins et al., in 1991, 34 cases of LCENC have been reported so far in literature, out of which 29 cases were associated with other epithelial cancer (Table/Fig-4) [1]. Ovarian cancer metastasizes to the cervix very rarely [2]. On an extensive literature review, we did not find any case of LCNEC of the ovary metastasizing to the cervix. Hence this may be the 1st case with metastasis to the cervix.
Clinico-pathological features of large cell neuroendocrine carcinoma of the ovary with metastasis reported in literature including the current case [4,5,7–12].
| Cases | Author | Age | Size (cm) | IHC Markers | Associated tumour | Metastasis | Stage | Treatment | Follow-up |
|---|
| 1. | Eichhorn et al., [7] | 58 | 30 | NA | Mucinous borderlinewith mucinous Adca | Serosa | IIIb | TAH, BSO, Ch | DOD 8 months |
| 2. | Chen [8] | 73 | 11 | NA | Mucinous Adca,p/h/o breast Ca | Multiplemetastasis | IIIc | BSO, Om,prior TAH, Ch | DOD 8 months |
| 3. | Choi et al., [5] | 71 | 6.5 | NA | Serous Adca | RP nodules | IIIb | TAH,BSO, Ch | NED 8 months |
| 5. | Tsuji et al., [4] | 46 | 15 | CA-125, LDH, NSE | Dermoid cyst | Multiplemetastasis | IIIc | Sub-TAH, BSO,Om, Ch | DOD 4 months |
| 6. | Tartaglia et al., [9] | NA | NA | CA125 Normal | None | Endometrium | NA | NA | NA |
| 7. | Draganova-Tachevaet al., [10] | 68 | 7 | CA-125 | Serous Adcawith mucin | Multiplemetastasis | IV | TAH, BSO, Om,debulking, Ch | DOD 7 months |
| 8. | Chenevert et al., [11] | 53 | 20 | CA-125, CEA, LDH | Mucinous Adca+ teratoma | Multiplemetastasis | IV | TAH BSO, Om,LN, Ch | DOD 3 months |
| 10. | Aslam et al., [12] | 76 | 30 | CA-125 Normal | None | PD | IIb | TAH, BSO, Om, | DOD post op |
| 11. | Present case | 36 | 6 | CA-125, CEA | None | Cervix, LN | IIIc | TAH, BSO | AWD 3 months |
TAH: total abdominal hysterectomy; BSO: bilateral salpingio-ophorectomy; Om: omentectomy; DOD: died of disease; AWD: alive with disease; NA: not available; post op: post operatively; Adca: adenocarcinoma; LN: lymph nodes; M: mediastinal; RP: retroperitoneal; PD: pouch of Douglas; Ch: chemotherapy; p/h/o: past history of; ca: carcinoma.
The tumours are solid-cystic in almost all cases. The most common marker elevated was CA-125, followed by CEA which was same in our case. Histologically, the tumour cells are usually arranged in solid sheets, cords, trabeculae and nests, some showing the follicle-like spaces with peripheral palisading, also described as cribriform pattern by some authors (as seen in our case) [1,3]. Individual tumour cells are intermediate to large sized with moderate to abundant eosinophilic granular cytoplasm and round to oval vesicular nuclei with prominent nucleoli in some cases or possessing coarsely stippled chromatin without prominent nucleoli in others [1,3,4]. Necrosis is also frequent, in some cases showing large areas of geographic necrosis, comedo like necrosis or single cell necrosis [3–5]. Immunohistochemically, the tumour cells are immunopositive for pan-cytokeratin, chromogranin A, synaptophysin, Neuron Specific Enolase (NSE), Epithelial Membrane Antigen (EMA), CK7, CAM 5.2, and in some cases for CD56, and c-kit (12). MIB-1 labelling index is usually very high [1].
In our case, the most important differential diagnosis considered was adult granulosa cell tumour and sertoli cell tumour due to the organoid architecture, Call-Exner body like follicular pattern and peripheral tumour cell palisading. Granulosa cell tumour shows prominent nuclear grooves which were absent in our case. Sertoli cell tumours such as Sex Cord Tumour with Annular Tubules (SCAT) with similar architecture show presence of hyaline bodies within the islands and bland nuclei. Immunohistochemistry conclusively resolved the issue by showing negativity for inhibin, CD99 (MIC-2) and vimentin which are generally positive in all sex-cord stromal ovarian tumours [5], and positivity for the neuroendocrine and epithelial markers. The metastasis of a neuroendocrine carcinoma to the ovary from other sites such as lungs, gall bladder, bowel etc. was excluded by clinical and radiological evaluation. The possibility of ovarian metastasis from primary neuroendocrine carcinoma of the cervix was ruled out due to raised serum CA-125, unilateral ovarian involvement, absence of ovarian surface involvement and absence of grossly evident tumour in the cervix [1,2]. Moreover, LCNEC of ovary are more commonly positive for pan-cytokeratin and negative for MIC-2 while the opposite is true of the cervix LCNEC [6]. Metastasis of ovarian tumours to cervix without any contiguous spread is very rare and the reason for this remains obscure. However, as such cases usually show spread to intrabdominal lymph nodes, it has been postulated that cervical metastasis arise from retrograde lymphatic spread as there is rich interconnection of the lymphatic drainage of the genital tract [2].
Conclusion
We report here a rare case of pure large cell neuroendocrine carcinoma of the ovary and the first case with metastasis to the cervix. Though rare, they should be considered in the differential diagnosis of tumours showing nests and trabeculae and confirmed by immunohistochemistry. Possibility of metastasis should be excluded before labelling a tumour as primary ovarian LCNEC.
TAH: total abdominal hysterectomy; BSO: bilateral salpingio-ophorectomy; Om: omentectomy; DOD: died of disease; AWD: alive with disease; NA: not available; post op: post operatively; Adca: adenocarcinoma; LN: lymph nodes; M: mediastinal; RP: retroperitoneal; PD: pouch of Douglas; Ch: chemotherapy; p/h/o: past history of; ca: carcinoma.
[1]. Collins RJ, Cheung A, Ngan HYS, Primary mixed neuroendocrine carcinoma of the ovaryArch Gynecol Obstet 1991 248:139-43. [Google Scholar]
[2]. Fox H, Agrawal K, Langley FA, A clinicopathologic study of 92 cases of granulosa cell tumour of the ovary with special reference to the factors influencing prognosisCancer 1975 35(1):231-41. [Google Scholar]
[3]. Veras E, Deavers MT, Silva EG, Malpica A, Ovarian non-small cell neuroendocrine carcinoma: a clinicopathologic and immunohistochemical study of 11 casesAm J Surg Pathol 2007 31(5):774-82. [Google Scholar]
[4]. Tsuji T, Togami S, Shintomo N, Fukamachi N, Douchi T, Taguchi S, Ovarian large cell neuroendocrine carcinomaJ Obstet Gynaecol Res 2008 34(4 Pt 2):726-30. [Google Scholar]
[5]. Choi YD, Lee JS, Choi C, Park CS, Nam JH, Ovarian neuroendocrine carcinoma, non-small cell type, associated with serous carcinomaGynecol Oncol 2007 104(3):747-52. [Google Scholar]
[6]. McCluggage WG, Young RH, Immunohistochemistry as a diagnostic aid in the evaluation of ovarian tumoursSemin Diagn Pathol 2005 22(1):3-32. [Google Scholar]
[7]. Eichorn JH, Young RH, Neuroendocrine Tumours of the Genital TractAm J Clin Pathol 2001 115(Suppl 1):S94-S112. [Google Scholar]
[8]. Chen KTK, Composite large-cell neuroendocrine carcinoma and surface epithelial stromal neoplasm of the ovaryInt J Surg Pathol 2000 8:169-74. [Google Scholar]
[9]. Tartaglia E, Di Serio C, Rotondi M, Di Serio M, Scaffa C, Tolino A, Endometrial metastasis of a primitive neuroendocrine ovarian carcinoma: management and treatment of a caseEur J Gynaecol Oncol 2008 29(1):101-04. [Google Scholar]
[10]. Draganova-Tacheva RA, Khurana JS, Huang Y, Hernandez E, Zhang X, Large cell neuroendocrine carcinoma of the ovary associated with serous carcinoma with mucin production: a case report and literature reviewInt J Clin Exp Pathol 2009 2(3):304-09. [Google Scholar]
[11]. Chênevert J, Bessette P, Plante M, Têtu B, Dubé V, Mixed ovarian large cell neuroendocrine carcinoma, mucinous adenocarcinoma, and teratoma: a report of two cases and review of the literaturePathol Res Pract 2009 205(9):657-61. [Google Scholar]
[12]. Aslam MF, Choi C, Khulpateea N, Neuroendocrine tumour of the ovaryJ Obstet Gynaecol 2009 29(5):449-51. [Google Scholar]