Antibiotic resistance is increasing at alarming levels and has emerged as a major public health concern of the 21st century [1]. β-lactams are among the commonly used classes of antibiotics. However, resistance to β-lactams has also emerged and production of β-lactamases is the most common cause of resistance to these drugs [2]. To counter the effect of β-lactamases, penicillinase resistant penicillins and first generation cephalosporins were introduced during the 1960s. This remained the mainstay of therapy for about 20 years, before the resistance due to β-lactamases produced by Gram negative bacteria became a serious problem. To meet this threat extended spectrum cephalosporins were introduced during the late 1970s [3]. Subsequently, a group of β-lactamases were discovered in Germany [4] that hydrolyzed extended spectrum cephalosporins and were named Extended Spectrum Beta- Lactamases (ESBL).
There is no consensus regarding the definition of ESBLs. ESBLs may be defined as a group of enzymes that are capable of conferring resistance to penicillins, first, second and third generation cephalosporins and aztreonam (but not cephamycins and carbapenems) and render them ineffective [5]. ESBLs are transmissible β-lactamases which are inhibited by clavulanic acid, tazobactam or sulbactam, and which are encoded by genes that can be exchanged between bacteria [6]. Majority of the ESBLs are found in Klebsiella spp. and Escherichia coli of the Enterobacteriaceae family [7,8].
Risk factors for infection with ESBL producing organisms are prolonged antibiotic usage, ICU stay, recent invasive procedure, pressure sores, anaemia and permanent urinary catheter [9]. Effective and rational usage of antibiotics in ICUs is important for prevention of development of antibiotic resistance.
ESBL producing strains remain undetected as they are difficult to detect by routine susceptibility testing methods and may show false susceptibility to antibiotics by Kirby- Bauer disc diffusion methods [10]. ESBL detection is important as knowledge about its prevalence is helpful to formulate infection control measures and to prevent their spread [10].
Praduyumna Bal Memorial Hospital (PBMH), the hospital wing of Kalinga Institute of Medical Sciences (KIMS), is a 1500 bedded tertiary care hospital and is equipped with state of the art ICUs. No study has been done in this area to determine the prevalence of ESBL producing Escherichia coli in the ICUs. Hence this study was undertaken to document the prevalence and resistance pattern of ESBL producing Escherichia coli and to help in implementing an effective antibiotic policy.
Materials and Methods
This is a cross sectional study conducted over a period of 4 years (Sept 2011 to Sept 2015) in the Department of Microbiology, Kalinga Institute of Medical Sciences, Bhubaneswar. Consecutive non-duplicate isolates of E.coli recovered from clinical samples of patients admitted to different ICUs of KIMS i.e., general ICU, paediatric ICU, neonatal ICU and medicine ICU were included. The samples (urine, pus, blood, endotracheal aspirate, sputum, catheter tip, high vaginal swab, body fluids) were collected according to standard procedures [11] and transported without delay. A total of 6800 samples were collected, from which consecutive, non repetitive E.coli isolates were obtained from 445 males and 593 females, across all age groups. All the clinical isolates other than E.coli were excluded. Ethical approval was obtained from the Ethical committee of the institute and informed consent was obtained from patients. All samples were inoculated onto blood agar and Mac Conkey agar except urine which was inoculated onto CLED (Cysteine Lactose Electrolyte Deficient) agar and incubated aerobically at 37°C. Bacterial pathogens were identified as per the standard protocol [12,13]. All Escherichia coli isolates were subjected to routine antimicrobial susceptibility testing by Kirby-Bauer diskc diffusion method according to CLSI guidelines [14]. The commercially available discs were procured from Himedia labs. According to CLSI guidelines [11] the following discs were used for antibiotic susceptibility testing of E.coli isolates - ampicillin (AMP, 10μg), amikacin (AK, 30 μg), gentamycin (GEN,10 μg), ciprofloxacin (CIP, 5 μg), ofloxacin (OF, 5 μg), levofloxacin (LE, 5 μg), cefuroxime (CXM, 30 μg), cefixime (CFM, 30 μg), cefpodoxime (CPD,10 μg), ceftriaxone (CTR,30 μg), cefotaxime (CTX, 30μg), ceftazidime (CAZ,30 μg), cefipime (CPM,30 μg), aztreonam (AT,30 μg), cotrimoxazole (COT, 1.25/23.75 μg) amoxyclav (AMC,20/10 μg), piperacillin-tazobactum (PIT, 100/10 μg), imipenem (IPM, 10 μg) norfloxacin (NX,10 μg), nitrofurantoin (NIT,300 μg). Norfloxacin and nitrofurantoin were tested against urinary isolates only. The quality control of antibiotic sensitivity was done using E. coli ATCC 25922 and E. coli ATCC 35218 (for β-lactam/β-lactamase inhibitor combination) [11].
Screening Test for ESBLs
An inoculum of 0.5 Mc Farland standard turbidity was prepared on a nutrient broth from an isolated E.coli colony taken from 18-24 hour agar plates. A sterile swab was dipped into the nutrient broth within 15 minutes of preparing the inoculum and inoculated onto a dried and sterile Mueller Hinton Agar (MHA) plate. The antibiotics were applied to the surface of the plate after 3-5 minutes of inoculation. The discs were pressed firmly against the surface of the plate and distributed evenly so that the minimum distance between the discs was 24 mm. The plates were inverted and incubated aerobically at 37°C overnight [10].
If any of the isolates had zone of inhibition for 3rd generation cephalosporins i.e., Cefpodoxime (10μg)≤ 17mm, ceftazidime (30 μg) ≤ 22mm, aztreonam (30μg) ≤ 27mm, cefotaxime (30μg) ≤27mm and ceftriaxone (30μg) ≤ 25mm (ESBL breaking point) as well as strains which were resistant were taken as screen positive for ESBL [14].
All strains found to be ESBL screen test positive were subjected to further confirmation by CLSI recommended phenotypic confirmatory tests [14].
Phenotypic Confirmatory Disc Diffusion Test (PCDDT) [
14]
The strains screened positive for ESBL production were tested by PCDDT for confirmation. Discs of ceftazidime (CAZ-30μg) and ceftazidime with clavulanic acid (CAC-30/10μg) and cefotaxime (CTX- 30μg) and cefotaxime-clavulanic acid (CTX/C-30/10μg) were dispensed at a minimum distance of 24mm on an MHA agar plate inoculated with the lawn culture of the E.coli isolate screened positive for ESBL production and incubated aerobically at 37°C overnight. If there was an increase in zone size by ≥ 5mm with ceftazidime/clavulanic acid and cefotaxime-clavulanic acid in comparison to ceftazidime or cefotaxime alone, then the strain of Escherichia coli was confirmed to be an ESBL producer [Table/Fig-1].
Enhancement of zones of inhibition by > 5mm of discs containing ceftazidime + clavulanic acid and cefotaxime + clavulanic acid as compared to ceftazidime and cefotaxime alone.
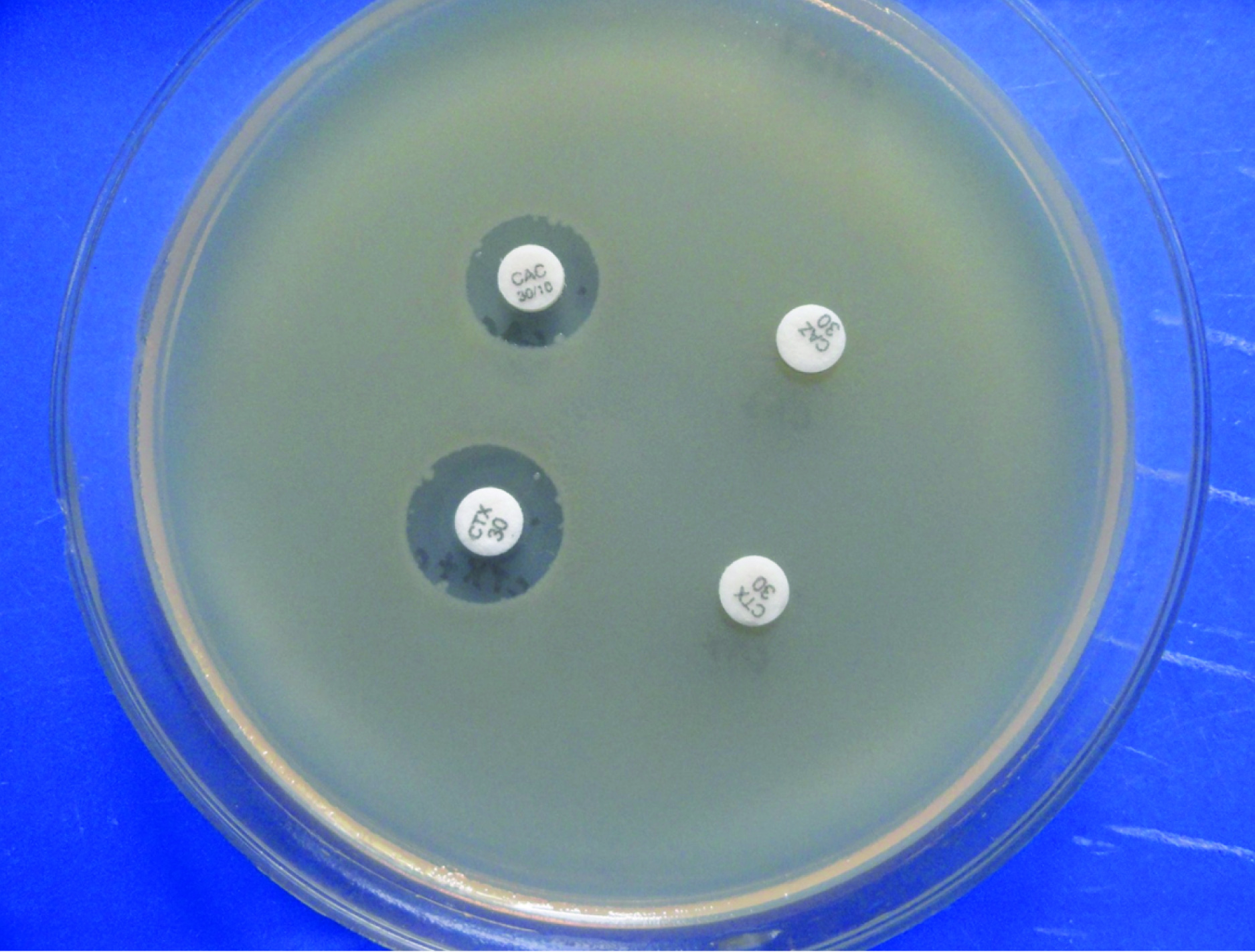
E. coli-ATCC 25922 and Klebsiella pneumoniae-ATCC 700603 were taken as controls for the phenotypic confirmatory test for ESBL [14].
Statistical Analysis
Proportions were compared using Chi-Square test to determine the significance of factors influencing acquisition of ESBL producing strains. Difference was considered significant if p-value was < 0.05.
Results
A total of 1038 E.coli isolates were isolated from 6800 different clinical samples. Maximum number of E.coli were isolated from urine, 859 (82.8%) followed by pus, 90 (8.7%) and blood 35(3.4%) [Table/Fig-2]. In the screen test for ESBL production, 452 were positive and 276 isolates were confirmed to be ESBL producers [Table/Fig-3]. Prevalence of ESBL producers among male patients was 59.3% and 62.4% among female patients. It was found out that there was no statistically significant association among sex of the patients with ESBL producing organisms (p-value =0.7, p > 0.05) [Table/Fig-4]. Prevalence of ESBLs was maximum in Paediatric ICU (80.9%) and least in coronary ICU (38.5%) [Table/Fig-5]. Among the 276 ESBL producers, 228 (82.6%) were from urine samples followed by pus (9.8%) and the least was from CSF (0). This association between ESBL production and urine samples collected from patients was statistically significant (p <0.05). Antimicrobial susceptibility among ESBL producing E.coli showed that the most effective antibiotic for ESBL producers was imipenem (96.7% sensitive), followed by amikacin (88.4%) and piperacillin-tazobactum (87%). The least sensitive was ampicillin (100% resistant). Among urinary isolates, nitrofurantoin was the most sensitive drug (83.6%) [Table/Fig-6].
Distribution of Escherichia coli in various samples.
| Serial no. | Samples | E.coli isolatedNo. (%)n=1038 |
|---|
| 1. | Urine | 859 (82.8%) |
| 2. | Pus | 90 (8.7%) |
| 3. | Blood | 35(3.4%) |
| 4. | Endotracheal aspirate | 20(1.9%) |
| 5. | Sputum | 10(0.9%) |
| 6. | CSF | 10(0.9%) |
| 7. | High vaginal swab | 6(0.6%) |
| 8. | Catheter tip | 5(0.5%) |
| 9. | Others (bile, bone marrow, vitreous fluid) | 3(0.3%) |
| TOTAL | 1038(100%) |
ESBL positive Escherichia coli.
| Isolates | Number (%) |
|---|
| Total no. of Escherichia coli isolates | 1038 |
| Escherichia coli resistant to 3rd generation cephalosporins | 452 |
| ESBL positive Escherichia coli | 276 (61.1%) |
Distribution of ESBL strains among male and female patients
| Sex | ESBLN=276 | Non-ESBLN = 176 | Total |
|---|
| Male | 115 (59.3%) | 79 | 194 |
| Female | 161(62.4%) | 97 | 258 |
Distribution of ESBL isolates among various ICUs.
| Sl. No. | Type of ICU | Samples screened | ESBL positiveno. (%) |
|---|
| 1. | General ICU | 157 | 98 (62.4%) |
| 2. | Paediatric ICU | 115 | 93(80.9%) |
| 3. | Neonatal ICU | 85 | 35 (41.2%) |
| 4. | Medicine ICU | 82 | 45 (54.9%) |
| 5. | Coronary ICU | 13 | 5 (38.5%) |
| Total | 452 | 276 (61.1%) |
Antibiogram of ESBL producing Escherichia coli.
| Antibiotics | SensitiveNo.(%) | ResistantNo. (%) |
|---|
| Imipenem | 267 (96.7%) | 9 (3.3%) |
| Amikacin | 244 (88.4%) | 32 (11.6%) |
| Piperacillin- Tazobactam | 240 (87%) | 36 (13%) |
| Gentamicin | 174 (63%) | 102 (37%) |
| Cefuroxime | 34 (12.3%) | 242 (87.7%) |
| Cefotaxime | 31 (11.2%) | 245 (88.8%) |
| Cefixime | 36 (13%) | 240 (87%) |
| Ceftazidime | 104 (37.9%) | 172 (62.1%) |
| Ceftriaxone | 57(20.7%) | 219 (79.3%) |
| Cefpodoxime | 10 (3.6%) | 266 (96.4%) |
| Cefipime | 174 (63.1%) | 102 (36.9%) |
| Aztreonam | 17 (6.2%) | 259 (93.8%) |
| Ciprofloxacin | 48 (17.4%) | 228 (82.6%) |
| Ofloxacin | 58 (21%) | 218 (79%) |
| Levofloxacin | 74 (26.8%) | 202 (73.2%) |
| Amoxyclav | 34 (12.3%) | 242 (87.7%) |
| Cotrimoxazole | 38 (13.8%) | 238 (86.2%) |
| Norfloxacin * | 42 (18.1%) | 190 (81.9%) |
| Nitrofurantoin * | 194 (83.6%) | 38 (16.4%) |
| Ampicillin | 0 | 276 (100%) |
(*only in urinary isolates, n= 232.)
Discussion
Infections by ESBL producing organisms have emerged as a major problem and the failure of therapy with broad spectrum antibiotics are creating serious problems [15]. Misuse/overuse of antimicrobials in ICUs will not only be expensive but also cause unforeseen menace of drug resistance in future as ICU patients are more susceptible to infection and colonization by various pathogens [16].
In our study, out of a total of 6800 clinical samples, the number of isolates of Escherichia coli was 1038 (15.2%). This in contrast to the Euro surveillance study where the rate of isolation of E.coli from all clinical samples was comparatively higher (39.8%) [17]. E.coli was isolated mostly from urine (82.8%) samples. Other workers elsewhere reported isolation rate of E.coli from urine to be 15.6% (2004), 49.8% (2004) and 20.4% (2012) [18–20] [Table/Fig-7].
Comparison of findings of our study with other studies.
| Characteristics compared | Results of our study | Results of the studies of other workers |
|---|
| Rate of isolation of E.coli from various samples | 15.2% | 39.8% {Kronenberg et al., [17]} |
| Isolation of E.coli from urine samples | 82.8% | 15.6% {Babypadmini et al., [18]}49.8% {Tankhiwale et al., [19]}20.4% {Rajan et al., [20]} |
| Prevalence of E.coli resistant to 3rd generation cephalosporins | 44% | 5.8% {Kronenberg et al., [17]} |
| Prevalence of ESBL producing E.coli from ICUs | 61% | 12.8% {Zhanel et al., [22]}70.6% {Ashrafian et al., [23]}10.3% {Rath et al., [24]}80% {Shanthi et al., [25]} |
| Highest prevalence of ESBL producing E.coli among various ICUs | Paediatric ICU : 80.9% | Paediatric ICU: 50% {Lenhard-Vidal et al., [29])} |
| ESBL producers among urinary isolates of E.coli | 63.5% | 31.6% {Chatterjee et al., [31]} |
| Resistance to antibioticsCefotaximeCeftriaxoneCeftazidimeOfloxacinNitrofurantoinAmoxy-clav | 89%79%62%79%16%88% | 97%95%29%19%1%8% {Stoesser et al., [32]} |
Studies from Switzerland (Kronenberg et al.,) reported the prevalence of E.coli resistant to 3rd generation cephalosporins to be 5.8% in Europe whereas 44% of E.coli was resistant to 3rd generation cephalosporins in our study [17] [Table/Fig-7]. The high prevalence of E.coli resistant to 3rd generation cephalosporins in our study may be due to over reliance on third generation cephalosporins to treat Gram negative infections and lack of regulated hospital antibiotic policy in our country [21].
The Canadian National Intensive Care Unit (CAN-ICU) study was the first to document that ESBL-producing E.coli are becoming more common than ESBL-producing Klebsiella spp. in ICUs [22]. Detection rate of ESBL positive E.coli in ICUs varies greatly in different parts of the globe as well as across India, ranging from 12.8% in Canada [22] to as high as 70.6% in Iran [23]. The prevalence of ESBL producing E.coli among in ICUs in India ranges from 10.35% [24] to 80% [25], whereas the prevalence rate in our study is 61% [Table/Fig-7].
Although some studies report male sex to be a risk factor for ESBL production [26,27], our study corroborates with the study conducted by Nibedita Das et al., where there was no significant association between ESBL production and male sex [28].
In our study, among the various ICUs, the highest prevalence of ESBL producers was in the paediatric ICU (80.9%). This finding is in accordance with the study conducted by Vidal et al., in Brazil, where the prevalence of ESBL Escherichia coli was highest in paediatric ICU (50%) [29].
This study shows that 82.6% of ESBL producers were urinary isolates. This could be due to the fact that urinary tract infections are among the most common infections encountered in clinical practice [30]. The ESBL producers in urinary isolates in our study were 63.5% whereas it was 31.6% in a study conducted by Chatterjee et al., in 2012 [31] [Table/Fig-7].
In our study, the resistance to cefotaxime, ceftriaxone, ceftazidime, ofloxacin, nitrofurantoin and amoxicillin-clavulanic acid was 89%, 79%, 62%, 79%, 16% and 88% respectively, in comparison to the study by Stoesser et al., where the corresponding resistance rate was 97%, 95%, 29%, 19%, 1% and 8% [Table/Fig-7] [32]. High prevalence of co-resistance was observed against ciprofloxacin (83%), and co-trimoxazole (86%), that is also in accordance with other studies [33–35]. This concomitant antimicrobial resistance is common as ESBL production coexists with resistance to several other antibiotics [36]. The reason for this resistance is that ESBLs are encoded by plasmids, which also carry resistance genes for other antibiotics [37].
Multi-drug resistance may be due to a number of factors like inappropriate self-medication, lack of prescribing regulations, substandard or falsified medicines and agricultural use of antibiotics [38]. As ESBL producing Escherichia coli are becoming increasingly multidrug resistant, there will be great limitation over the choice of drugs for treating these patients [24].
Limitations
Genotypic characterization of the enzymes has not been done which would have helped in characterization of the genomic pattern of the ESBL enzymes in the community. It has been reported that in comparison to genotypic tests, phenotypic tests are highly sensitive and specific but phenotypic confirmatory tests may sometimes be falsely positive or negative [6].
Conclusion
The findings of this study emphasizes the need for a continuous surveillance in the ICUs to detect the resistant strains, strict guidelines for the antibiotic therapy and the implementation of infection control measures to reduce the increasing burden of antibiotic resistance. Knowledge of the resistance pattern of ESBL producing Escherichia coli in this geographical area will be helpful in formulating the antibiotic policy of KIMS hospital. It is recommended that along with conventional antibiogram, routine ESBL testing should be done.
(*only in urinary isolates, n= 232.)