In the chaos of life, any psychiatric disorder can often get neglected, aggravating the situation and leading to an illness requiring chronic treatment. When the symptoms start interfering in the daily activities of a person, the person requires attention and care [1].
In 1992, the International Classification of Diseases (ICD-10) introduced the concept of Mixed Anxiety-Depression Disorder (MAD) [2]. So drugs having properties to combat both anxiety and depression, with lesser side effects, might be useful for such clinical conditions [3].
Depression and anxiety disorders are the most common mental illnesses, suggested estimate to affect excess of 10-15% of the population at some times in their lives. Anxiety and depression are parts of almost every psychiatric disorder. Both disorders can have pharmacological treatments that have been developed [4]. Atypical antipsychotics have approval as adjunctive treatment for resistant major depressive disorder [5]. However, increased concern is expressed regarding the safety profile of these drugs [6].
Blonanserin is a novel atypical antipsychotic agent approved in Japan and Korea for the treatment of patients with schizophrenia based upon various trials [7–12]. It is approved in India in 2012 [13]. However, sufficient data regarding its antidepressant effect is not available.
However, detailed studies are required in order to definitively position Blonanserin with respect to other antipsychotic agents [17–19]. No conclusive study of its proven anxiolytic –antidepressant activity could be found. Some meta-analysis studies have pointed out a possible anxiolytic action but such specific study (with Blonanserin alone as a group) planned and executed could not be retrieved by us.
The present study was planned to investigate, if Blonanserin has antidepressant and antianxiety effect.
To study the effect of Blonanserin on anxiety and depression in animal models.
Materials and Methods
Study was carried out in 5 groups of rats (10 rats/group) of either sex for antianxiety and antidepressant tests at Department of Pharmacology and central animal house, Bharati Vidyapeeth Deemed University, Medical College and Hospital Sangli. The protocol and synopsis was discussed in IAEC (Institutional Animal Ethical Committee), including number of animals, reuse of animals and end point in each test. IAEC approved the project, after which it was started.
Animals: Male and female (non-pregnant) Wistar rats weighing around 200-250g were used. They were housed under standardized conditions and fed with standardized pellet diet. Water was allowed along with adlibitum. Experiments were conducted between 09:00 AM to 4:00 PM. All animal procedures were carried out in accordance with the SOPs approved by IAEC as per recommendations of CPCSEA guidelines.
Drugs’ dosing: The clinical doses of various drugs were converted to rat equivalent doses using standardized formula [20]. The volume of the drug administered orally was <1ml/100g of a rat. All the drugs were freshly prepared on the day of the experiment and used on the same day. All the drugs and chemicals were purchased locally. Tab. Blonanserin (Elicia 4-Zydus Neurosciences), Tab. Diazepam (Valium 5 -Abbott Healthcare Pvt. Ltd.), Tab. Imipramine (Antidep 75-Torrent Pharmaceuticals).
Antianxiety study: Elevated plus maze (EPM) [21]: was used to study antianxiety effect of Blonanserin as described below.
Drug treatment schedule for antianxiety study (elevated plus maze).
| Group No | No of Rats (Total 50) | Treatment |
|---|
| I | 10 | Control (1% Gum Acacia) |
| II | 10 | Diazepam (1.5mg/kg) |
| II | 10 | Blonanserin (0.075mg/kg) |
| III | 10 | Blonanserin (0.2mg/kg) |
| IV | 10 | Blonanserin (0.8mg/kg) |
Test drugs were administered per orally (p.o.) according to body weight. Diazepam was administered 60 min prior while Blonanserin and 1% Gum Acacia (10 ml/kg) were administered 90 min prior to behavioural observations in test. The behavioural performances recorded during a 5 min test period were; number of entries into and time spent in open and closed arm. Entry into an arm was considered valid only when all four paws of the rat were inside that arm. The apparatus was thoroughly cleaned with 10% ethanol after each trial.
Percentage of Open Arm Entries (%OAE) which indicates exploratory behaviour and is one of the parameters of antianxiety effect and Percentage of Time Spent in Open Arm (%TSOA) were calculated. At the end of the study, no animal showed any grave injury or disability.
Antidepressant study: Forced swimming test [22]: was used to test antidepressant effect of Blonanserin as described below.
| Group No | No of Rats (Total 50) | Treatment |
|---|
| I | 10 | Control (1% Gum Acacia) |
| II | 10 | Imipramine (60mg/kg) |
| II | 10 | Blonanserin (0.075mg/kg) |
| III | 10 | Blonanserin (0.2mg/kg) |
| IV | 10 | Blonanserin (0.8mg/kg) |
Drug treatment schedule for antidepressant study (Forced swimming test).
Test drugs were administered per orally (p.o.) according to body weight. Imipramine was administered 60 min prior while Blonanserin and 1% Gum Acacia (10 ml/kg) were administered 90 min prior to behavioural observations in test. After (60 or 90 min) drug administration, the rat was placed in the cylinder and immobility was measured for 5 min. A rat was judged to be immobile when it remained floating in the water in an upright position and only made very small movements necessary to keep its head above water. The total duration of immobility over the 5-min period was recorded. Five minutes later, the rats were removed to a 30°C drying room for 30 min. Water in cylinder was changed after each animal test.
During the test due care was taken to avoid any injury to animal. At the end of the study, no animal showed any grave injury or disability.
Statistical Analysis
Data was expressed as mean ± standard error of mean (SEM). Statistical analysis was carried out using one-way ANOVA (Analysis of variance) for significance between groups. The level of significance between individual groups was detected using unpaired t-test. For all tests effects with a probability of p < 0.05 considered to be significant.
Results
Antianxiety study
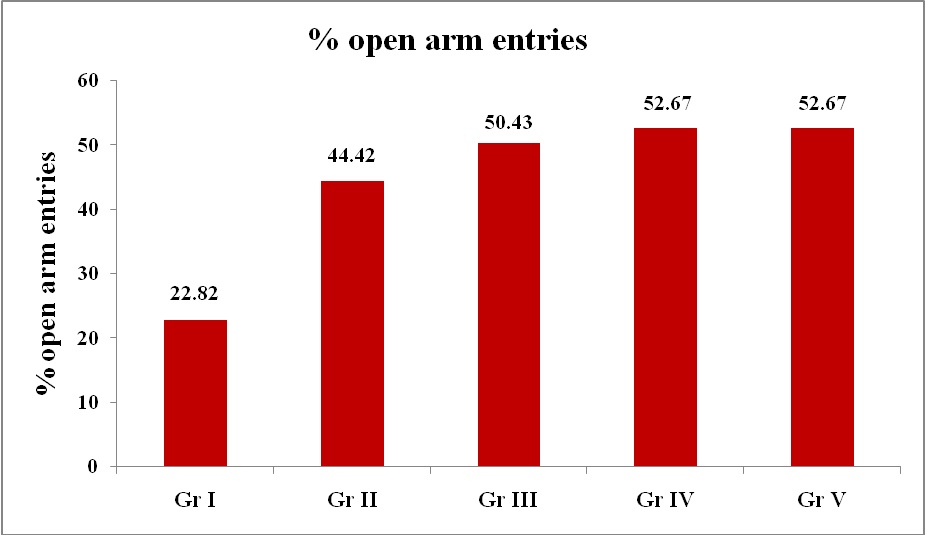
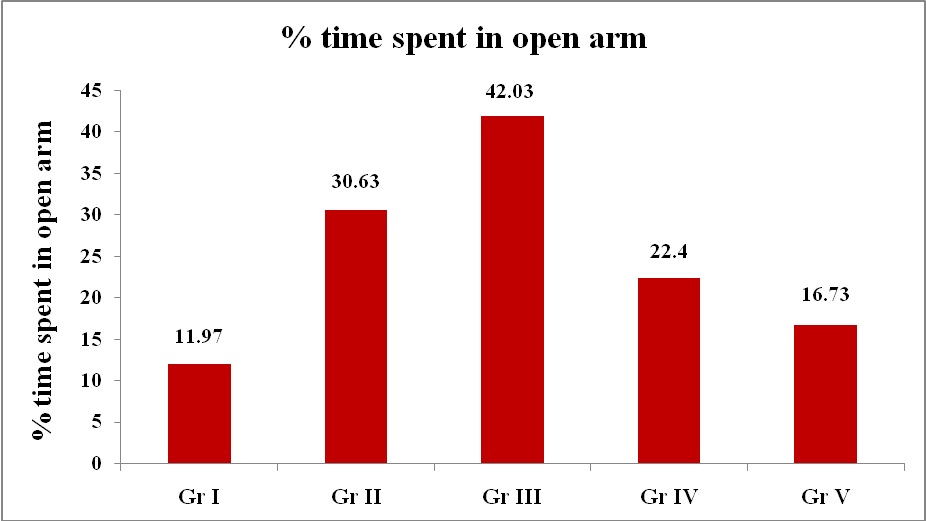
Elevated Plus Maze Test: There was statistically significant difference in means of % OAE of drug treated groups - Diazepam (1.5mg/kg), Blonanserin (0.075mg/kg), Blonanserin (0.2mg/kg) and Blonanserin (0.8mg/kg) when compared with control (1% Gum Acacia) [Table/Fig-1,2] indicating increased exploration of rats with Diazepam and Blonanserin. Indicating a comparable antianxiety effect by both drugs. Optimal effect of Blonanserin was observed with 0.075 mg/kg followed by 0.2mg/kg and 0.8mg/kg. For % time spent in open arms, 0.075mg/kg Blonanserin showed optimal effect as compared with other doses and comparable to diazepam [Table/Fig-3,4].
Effect of various treatments on behaviour of rats (% open arm entries) in elevated plus maze
| Group | Drug | Dose | Mean no. of arm entries | % open arm entries |
|---|
| Open | Closed | Total |
|---|
| I | Control (1% gum acacia) | 10 ml/kg | 1.9 ± 0.407 | 6.3 ± 0.396 | 8.2 ± 0.249 | 22.82 ± 4.892 |
| II | Diazepam | 1.5 mg/kg | 3.6 ± 0.163 | 4.5 ± 0.167 | 8.1 ± 0.179 | 44.42 ± 1.734 * |
| III | Blonanserin | 0.075mg/kg | 3.2 ± 0.327 | 3.1 ± 0.277 | 6.3 ± 0.472 | 50.43 ± 2.809 * |
| IV | Blonanserin | 0.2mg/kg | 2 ± 0.333 | 2 ± 0.537 | 4 ± 0.816 | 52.67 ± 3.096 *# |
| V | Blonanserin | 0.8mg/kg | 1.4 ± 0.221 | 1.2 ± 0.133 | 2.6 ± 0.305 | 52.67 ± 3.096 *# |
Data is expressed as Mean ± S.E.M.
p< 0.05 is significant
* = p < 0.05 when compared with control
# = p < 0.05 when compared with Diazepam
Percentage open arm entries in EPM test
%OAE = (open arm entries /total arm entries) × 100.
Gr I – Control (1% Gum Acacia – 10 ml/kg)
Gr II - Diazepam (1.5mg/kg)
Gr III – Blonanserin (0.075mg/kg)
Gr IV - Blonanserin (0.2mg/kg)
Gr V - Blonanserin (0.8mg/kg)
Effect of various treatments on behaviour of rats (% time spent in open arm) in elevated plus maze paradigm
| Group | Drug | dose | Mean Time spent (sec) | % time spent in open arm |
|---|
| Open arm | Closed arm |
|---|
| I | Control (1% Gum Acacia) | 10ml/kg | 35.9 ± 7.181 | 264.1 ± 7.181 | 11.97 ± 2.393 |
| II | Diazepam | 1.5mg/kg | 91.9 ± 5.759 | 208.1 ± 5.759 | 30.63 ± 1.919 * |
| III | Blonanserin | 0.075mg/kg | 126.1 ± 12.758 | 173.9 ± 12.758 | 42.03 ± 4.252 * # |
| IV | Blonanserin | 0.2mg/kg | 67.2 ± 8.601 | 232.8 ± 8.601 | 22.40 ± 2.867 * # @ |
| V | Blonanserin | 0.8mg/kg | 50.2 ± 7.801 | 249.8 ± 7.801 | 16.73 ± 2.599 |
Data is expressed as Mean ± S.E.M.
p < 0.05 is significant
* = p < 0.05 when compared with control
# = p < 0.05 when compared with Diazepam
@ = p < 0.05 when compared with Blonanserin (0.075 mg/kg)
Percentage time spent in open arm in EPM test
%TSOA = (time spent in open arm /total time) × 100
Gr I – Control (1% Gum Acacia – 10 ml/kg)
Gr II – Diazepam (1.5 mg/kg)
Gr III – Blonanserin (0.075 mg/kg)
Gr IV - Blonanserin (0.2 mg/kg)
Gr V - Blonanserin (0.8 mg/kg)
Antidepressant study
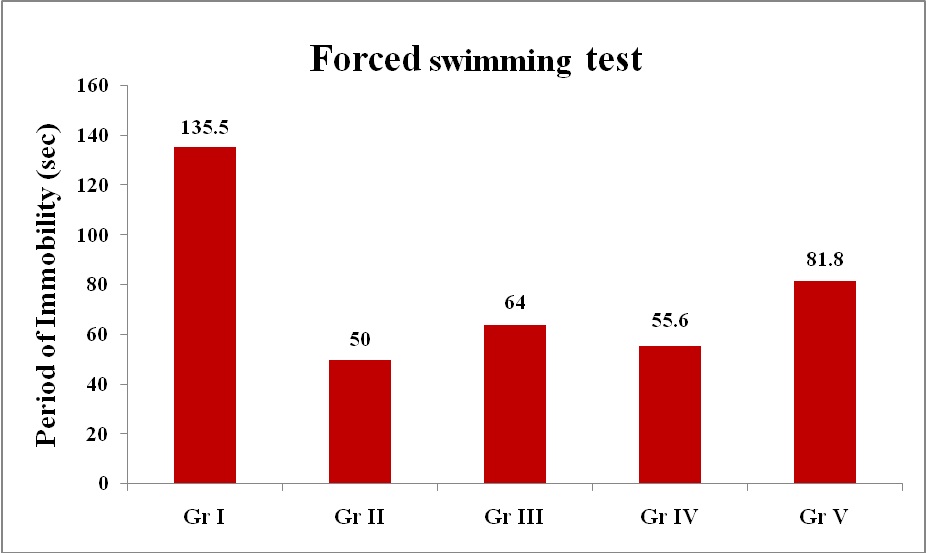
Forced Swimming Test: There was statistically significant difference in means of immobility time of drug treated groups Imipramine (60mg/kg), Blonanserin (0.075mg/kg), Blonanserin (0.2mg/kg) and Blonanserin (0.8mg/kg) when compared with control (1%gum acacia) indicating antidepressant effect of Imipramine and Blonanserin [Table/Fig-5]. Optimal effect was observed with 0.2mg/kg, followed by 0.075mg/kg and 0.8mg/kg of Blonanserin [Table/Fig-5,6].
Effect of various treatments on immobility time in forced swimming test
| Group | Drug | Dose | Mean Immobility time (sec) |
|---|
| I | Control(1% gum acacia) | 10 ml/kg | 135.5 ± 4.653 |
| II | Imipramine | 60 mg/kg | 50 ± 1.687* |
| III | Blonanserin | 0.075mg/kg | 64 ± 3.249* $ |
| IV | Blonanserin | 0.2 mg/kg | 55.6 ± 1.694* $ @ |
| V | Blonanserin | 0.8 mg/kg | 81.8 ± 4.197 * $ @ € |
Data is expressed as Mean ± S.E.M.
p < 0.05 is significant
* = p < 0.05 when compared with control
$ = p < 0.05 when compared with Imipramine (60 mg/kg)
@ = p < 0.05 when compared with Blonanserin (0.075 mg/kg)
€ = p < 0.05 when compared with Blonanserin (0.2 mg/kg)
Period of immobility (sec) in Forced swimming test.
Gr I – Control (1% Gum Acacia – 10 ml/kg)
Gr II – Imipramine (60 mg/kg)
Gr III – Blonanserin (0.075 mg/kg)
Gr IV - Blonanserin (0.2 mg/kg)
Gr V - Blonanserin (0.8 mg/kg)
Discussion
Blonanserin is an atypical antipsychotic drug. This agent has a high affinity, for receptors of dopamine D2 and serotonin 5-HT2A, higher for D2 than for 5-HT2A, which is different than other second generation atypical antipsychotic drugs. Blonanserin also has a low affinity for receptors of muscarine M1, histamine H1, adrenaline alpha1 and serotonin 5-HT2C [14]. Blonanserin has also affinity for 5-HT1A receptors where it shows indirect 5-HT1A partial agonistic activity [16].
Some studies have proven that systemic administration of Blonanserin increases extracellular levels of norepinephrine and dopamine, but not levels of 5-HT, glutamate, or gamma-aminobutyric acid in the prefrontal cortex. It also enhances neuronal activity in the locus coeruleus and ventral tegmental area without affecting activity in the dorsal raphe nucleus or the mediodorsal thalamic nucleus. The antagonistic properties of Blonanserin towards D2 and 5-HT2A receptors are postulated to contribute to increase in extracellular levels of dopamine and norepinephrine [23]. Some researchers have documented evidence of Blonanserin showing low inhibitory activity of neuronal re-uptake of noradrenaline, dopamine, serotonin [7]. Some reviewers have mentioned study with Positron Emission Tomography (PET) in healthy volunteers showing 80% striatal D2-like receptors occupancy by Blonanserin given in normal clinical doses [19].
Animal models of emotional disorders target to reproduce: 1) behavioural and physiological changes in a particular emotional state (face validity); 2) cause or aetiology (construct validity); 3) responses to treatment (predictive validity) as described by researchers and books [24]. Elevated plus maze and forced swimming test are acute, stress evoked tests for anxiety and depression respectively [25].
Elevated Plus-Maze
The elevated plus maze depends upon rodents’ inherent liking towards dark, enclosed spaces (or approach) and an unconditioned fear for heights/open spaces (or avoidance). It is a spontaneous rodent behaviour [26]. Provoked behaviour profiles in this test appear to include elements of neophobia, exploration and approach/avoidance conflict; thus, the apparatus is often referred to as an unconditioned spontaneous behavioural conflict model [27].
Open-arm avoidance is driven by an aversion to open spaces, leading to thigmotaxic behaviour, a natural reaction in which rats remain close to vertical surfaces. Earlier studies indicate that this behaviour is determined by two main factors, an exploratory drive which leads the rat to visit all parts of the maze and a fear drive which reduces the number of visits to aversive places and increases the number of visits to non-aversive places [28]. Benzodiazepine anxiolytics increase the proportion of activity in the open arms, whereas non-anxiolytic agents (e.g., amphetamine, caffeine) generally do not [29–32]. Researcher has reported that alprazolam like mixed anxiolytic and antidepressant drugs also show reliable anxiolytic effects in the elevated plus-maze test [33–36]. However, contradicting evidences are also present [37–40].
The role of 5-HT in anxiety is known. Antagonism at 5-HT2A and 5-HT2C receptors is involved in antianxiety effect of various drugs [22,41–44]. However, there are also reports of clear anxiolytic effects, or no anxiolytic effects, even after chronic administration [38]. A number of studies support the hypothesis that in the elevated plus-maze test high doses of 5-HT1A agonistic compounds are needed for their anxiolytic effects after chronic treatment [38].
In the present study, Blonanserin (0.075mg/kg) showed no significant difference in mean % OAE but significant increase in % time spent in open arm when compared with Diazepam (1.5mg/kg) which indicate that blonanserin (0.075mg/kg) has antianxiety effect. This antianxiety effect of blonanserin (0.075mg/kg) can be due to-Blocking of 5-HT2A and 5-HT2C receptors, Partial agonistic activity at 5-HT1A receptors, Inhibitory activity of neuronal re-uptake of serotonin and norepinephrine and increase in extracellular levels of norepinephrine as also expressed by earlier studies [23,45–47].
Antianxiety effect with higher dose of Blonanserin (0.2mg/kg and 0.8mg/kg) is not seen comparable to diazepam 1.5mg, may be because of increased levels of serotonin with increased dose of Blonanserin leading to sedation, (reduced no of arm entries and hence relative increase in % OAE but no significant comparable increase in % time spent in open arm) and masking the antianxiety effect.
Forced Swimming Test [
18,
22]
The Forced-Swimming Test is based on the tendency of animals to develop an immobile posture in an inescapable water cylinder. This immobility is considered as a passive stress-coping or depression-like behaviour (behavioural despair). Hence with antidepressant treatment, the animals are expected to actively perform escape-directed behaviours for longer duration than control animals (saline treatment). Porsolt’s modified FST is the most widely used tool, sensitive for screening of acute antidepressants [22].
It is also considered to provide a useful model to direct study for neurobiological and genetic mechanisms underlying stress and antidepressant responses [48,49]. The role of 5-HT1A receptors in depression has been evaluated by earlier studies. Researchers have reported an increase in postsynaptic 5-HT1A signaling, either by direct or indirect mechanisms in humans by main antidepressants [41].
Some post mortem studies also have reported that in suicide patients a reduction in number and binding affinity of 5-HT1A receptors is observed. This was also demonstrated using PET scanning analysis studies. Genetic studies in humans and 5-HT1A receptor knockout mice have supported that 5-HT1A receptor dysfunction may be an underlying mechanism in depressive disorders [50].
Norepinephrine (NE) is also of importance in the pathophysiology and treatment of depressive disorder as evaluated by earlier studies [51,52]. Evidences of importance of NE system are well aggregated by reviewers as follows. Functional biochemical differences observed in the NE system in postmortem brains between healthy controls and depressed patients were substantial. Genetic manipulation in animals leading to increase in NE neurotransmission reported to protect them from stress-induced depressive behaviour. Studies have reported that chemical depletion of NE increased the susceptibility of recovered depressed patients to a relapse and therapeutic agents specifically increasing NE activity act as effective antidepressants [51]. Some studies have reported that drugs acting simultaneously on 5-HT and NE neurotransmission may have superior antidepressant action [52].
One study has demonstrated Blonanserin attenuates the enhancement of immobility in the forced swimming test induced by repeated treatment with phencyclidine in mice [10].
In the present study, Blonanserin (0.2mg/kg) decreased immobility period significantly indicating its antidepressant effect. Blonanserin (0.075mg/kg) also showed antidepressant effect but less than that of Blonanserin (0.2mg/kg). Blonanserin (0.8mg/kg) did not show any antidepressant effect.
This effect of Blonanserin can be explained as -At lower doses (0.075 mg/kg) Blonanserin act more on 5HT2A/2C receptors as an antagonist and as dose is increased (0.2mg/kg) the antagonistic action on presynaptic 5-HT1A receptors may be seen which increases serotonin levels and alleviates depression. After further increase in dose (0.8mg/kg), serotonin levels may further be increased which lead to sedative action of Blonanserin so antidepressant effect is not seen with higher dose (0.8mg/kg). Inhibitory activity on neuronal re-uptake of dopamine, serotonin and norepinephrine and increased extracellular levels of norepinephrine and dopamine in prefrontal cortex may also be involved in its antidepressant effect as also expressed by earlier studies [23,45–47].
So, antianxiety and antidepressant effect of Blonanserin as evidenced in present study are likely to be mediated through an action on serotonin receptors and associated increase in norepinephrine and dopamine levels in brain as suggested by earlier researchers.
Limitation
We also acknowledge the limitation of our study that it is an animal study and hence results must be corroborated with human studies. Similarly being a study on psychiatric disorder individual animal or personal bias will also affect the outcome.
This potential and expectations has to be evaluated with larger clinical studies before brought into practice. However, results from the present study positively recommend planning of such studies.
Conclusion
Blonanserin is a newly developed, atypical antipsychotic drug. Many of the psychiatric disorders have comorbid symptoms of anxiety and depressions. This usually complicates the treatment of these disorders. A drug which is antipsychotic as well as antidepressant and/or antianxiety will be beneficial in such patients. Present study evaluated the potential of Blonanserin as an antianxiety and antidepressant drug. Our study demonstrated statistically significant antianxiety Effect of Blonanserin (comparable to diazepam). It also has shown potential to act as an antidepressant drug. Such a drug could be helpful in mixed psychiatric disorders (thought disorders accompanied by symptoms of anxiety and depression). Similarly, it may prove beneficial in reducing certain specific anxieties like neophobia.