The global demography is experiencing ageing that means process of rising in proportion of older people above 65 years of in the coming decades [1].
Many problems arise with increasing age, memory loss being one of the important ones. This may be due to Alzheimer’s disease or age related dementia. Memory enhancers, therefore, find important application in these patients.
Many Ayurvedic products which are being used traditionally for memory enhancement are ghruta or ghee based [2]. Goghruta (Cow Ghee) itself being used as a brain tonic to improve memory. It is said to promote all three aspects of mental functioning-learning, memory and recall [3]. The traditional texts also designate that cow ghee is a, Medhya Rasayana, beneficial for mental alertness and memory in adults as well in children [4,5].
However, there is a lack of scientific data regarding the effect of Cow Ghee on learning and memory. We have planned a study for authentication of traditional claims of cow ghee as a memory enhancer, using animal model.
According to studies conducted in the past, fatty acid analysis of ghee is found to have 60-70% saturated fat [6,7]. There has been concern about the possibility of ghee contributing to an increased risk of cardiovascular disease due to its high percentage of saturated fatty acids, leading to increased synthesis of cholesterol. Some previous studies have shown no effect or decreased LDL and increased HDL cholesterol but increased in weight [8,9].
Amongst all edible fats it is found that nutrition composition of cow ghee and butter is comparatively similar. The total content of fat (saturated, monounsaturated, polyunsaturated and cholesterol) is nearly the same [10] hence, we decide to take butter as a comparator arm and compare the effect of cow ghee and butter on memory and lipid profile.
Materials and Methods
The study was initiated after Institutional Animal Ethics Committee approval (Approval no. BVDUMC/3079/2015/001/006).
Preparation of Cow Ghee
Cow milk (2L approx.) was brought from the market. Cow Ghee was prepared from cow milk by natural Indian method [11]. Butter was brought from the market.
Dose
In Ayurveda science the recommended plain and medicated ghee drug dose is 40g/70 kg [12]. it has been extrapolated in animal [13] and used in rats
Experimental animals
Rats of either sex weighing 150-200g were used as experimental animals for study. Housing was done in standard cage (2 to 3 animals per cage). Animal coding was done according to standard protocol and animals were randomly allocated to four experimental groups as, Gr I control (Distilled water) treated group, Gr II was given Piracetam (25mg/100g), Gr III Cow Ghee (3.6g/kg) and Gr IV Butter (0.08mg/kg). All drugs were given by oral route.
Elevated plus-maze (EPM) model [14] was used for evaluation of extroceptive behaviour. On the first day, each rat was placed at the end of an open arm, facing away from the central platform and Transfer latency (TL) i.e., the time taken by rat with all its four legs to move into one of the closed arms was recorded. Even after 90s if the rats remained outside the closed arms they were pushed into one of the closed arm and TL considered as 90 secs. The rats were returned to cage after giving them another 90 secs to explore the maze [15]. Retention of this learned-task was examined 24 h after the first day trial. Drug treatment was given for 21 days. On the last day of therapy, 45 minutes prior to plus maze testing, diazepam was given in 1mg/kg intraperitoneally. TL (in seconds) was noted. The test was repeated 24 hours later.
Other model used to evaluate Introspective behaviour in rats was Morris water maze (MWM) Test [16].
The water maze apparatus consists of a circular water tank of 1.83 meters in diameter, divided into 4 quadrants. There was a 4 inch X 4 inch size escape platform submerged in one of the quadrant, the target quadrant. The top surface of the platform was hidden approximately 1 cm below the surface of the water. The pool was filled with water at a temperature of 18-26o C to a depth of about 40 cm. Chalk powder was added to the water just before the experiment to make water opaque. Permanently positioned distinctive objects were placed for facilitating spatial orientation of the animal. Positions of the cues were kept unchanged throughout the period of training.
In this test, rats learned to swim in a water tank to find an escape platform hidden under water. There were no obvious clues inside the water tank to mark the location of the platform so provisions should be made to configure clues outside the tank. Shorter escape latencies and shorter length of the path to find the platform reflected the learning abilities of these animals [17,18]. The experiment consisted of two parts, Acquisition trial and Retrieval trial.
In the acquisition part, consecutive trials on each day were conducted for four times with 5 min interval. Animals were supposed to escape to the platform hidden under the opaque water and could remain there for a duration of 20 sec [19]. In case of the inability of the animal to locate the hidden platform within 90 sec, he was gently guided by hand to the platform and allowed to remain there for 20 sec. Escape Latency Time (ESL) to locate the hidden platform in water maze was noted as an index of acquisition and learning. Starting position on each day to conduct four acquisition trials was changed daily on day four a probe test was performed. In this trial, the platform was removed and each animal was allowed to explore the pool for 90 sec. The time taken by the animal to reach the target quadrant (Escape Latency) and time spent in the target quadrant was measured. Greater latency to reach the target quadrant and less time spent in the target quadrant suggested memory impairment.
The drugs were given for 21 days. The retained memory was tested as above after three week. The initial and post treatment weight of rats were measured and noted in all groups. Blood was collected from median orbital plexus for measuring lipid profile like total cholesterol (TC), High Density Lipoproteins (HDL), Low Density Lipoprteins (LDL), Triglycerides and Very Low Density Lipoproteins (VLDL) both, before and after treatment for all groups.
Statistical Analysis
All the results are expressed as Mean ± S.E.M. Data was analysed by one-way analysis of variance (ANOVA) followed by Dunnett’s post-hoc test in Graph Pad Prism version 5.0. The p< 0.05 was considered as significant.
Results
Comparison of Lipid Profile Shown in [Table/Fig-1,2,3,4,5 and 6]
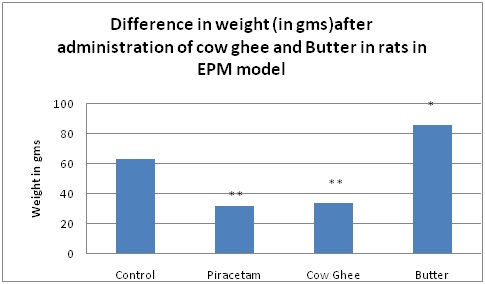
Difference in weight (ingms) after administration of Cow Ghee and butter in rats in elevated plus maze (epm) model.
Results are given as mean ± SEM of six animals in each group. Control group is compared with rest of the treated groups by one-way ANNOVA followed by Dunnett’s test. Significance at *p < 0.05; **p < 0.01; ***p < 0.001
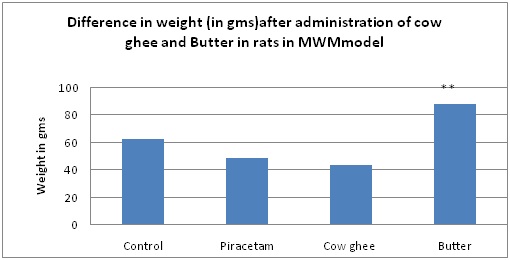
Difference in weight (ingms) after administration of Cow Ghee and butter in rats in morris water maze (MWM) model.
Results are given as mean ± SEM of six animals in each group. Control group is compared with rest of the treated groups by one-way ANNOVA followed by Dunnett’s test. Significance at *p < 0.05; **p < 0.01; ***p < 0.001
Effect of drug on escape latency of rats after treatment in EPM model.
| Group (N=6) | Mean± SEM | Significance | 95% confidence interval |
|---|
| Control | 27.67±6.756 | | 10.3-45.03 |
| Piracetam | 4±4.258 | * | -6.946-14.95 |
| Cow Ghee | 11.33± 7.069 | ns | -6.839-29.51 |
| Butter | 16.67±4.631 | ns | 4.763-28.57 |
Results are given as mean ± SEM of six animals in each group.
Control group is compared with rest of the treated groups by one-way ANNOVA followed by Dunnett’s test. Significance at *p < 0.05; **p < 0.01; ***p < 0.001
Effect of drug on transfer latency of rats after treatment in MWM model.
| Group (N=6) | Mean± SEM | Significance | 95% confidence interval |
|---|
| Control | 9.833 ±0.9098 | | 7.495-12.17 |
| Piracetam | 5.500 ±1.057 | * | 2.784-8.216 |
| Cow Ghee | 11.17 ±0.9804 | ns | 8.647-13.69 |
| Butter | 10.67 ±1.677 | ns | 6.382-14.95 |
Results are given as mean ± SEM of six animals in each group.
Control group is compared with rest of the treated groups by one-way ANNOVA followed by Dunnett’s test. Significance at *p < 0.05; **p < 0.01; ***p < 0.001
Effect of drug on lipid profile of rats after treatment in EPM model.
| Drug | Total CHL | HDL | TG | LDL | VLDL |
|---|
| Control | 113.5 ± 3.16 | 29.67 ± 1.599 | 87.67 ± 2.692 | 69 ± 4.64 | 16.5± 0.7638 |
| Piracetam | 110.5 ± 3.421 | 36 ± 1.327 | 87 ± 6.491 | 60.67 ± 6.265 | 17± 1.366 |
| Cow Ghee | 121.7 ± 4.246 | 34.17 ± 1.71 | 102.5 ± 7.619 | 66.83 ± 6.77 | 20.67± 1.453 |
| Butter | 119.3 ± 4.01 | 34.11 ± 1.5 | 115.3 ± 4.978** | 77.67 ± 0.9545 | 23.17± 0.9458** |
Results are given as mean ± SEM of six animals in each group.
Control group is compared with rest of the treated groups by one-way ANNOVA followed by Dunnett’s test. Significance at *p < 0.05; **p< 0.01; ***p < 0.001
Effect of drug on lipid profile of rats after treatment in MWM model.
| Drug | Total CHL | HDL | TG | LDL | VLDL |
|---|
| Control | 122.3± 5.829 | 34.33± 1.909 | 94.17± 4.629 | 69± 5.922 | 18.67± 0.9189 |
| Piracetam | 115.2± 5.648 | 33.33± 1.8921 | 96.5± 3.096 | 62.5± 6.12 | 19.33± 0.6667 |
| Cow Ghee | 117.5± 4.648 | 34.83± 0.9458 | 108± 3.357* | 61.67± 4.667 | 21.33± 0.6146* |
| Butter | 111.5± 4.945 | 35.5± 1.607 | 99.55± 117.5* | 56.17± 3.628 | 21.33± 0.7149* |
Results are given as mean ± SEM of six animals in each group.
Control group is compared with rest of the treated groups by one-way ANNOVA followed by Dunnett’s test. Significance at *p< 0.05; **p < 0.01; ***p< 0.001
Discussion
Effect of cow ghee and butter on Memory
Many experimental models are currently available for the evaluation of agents that affect learning and memory process. We used EPM for assessing short term memory [14,15] and MWM for long term memory [16,17] to assess nootropic activity of cow ghee and butter.
In an EPM model, decrease in TL which indicate increase in memory was seen only in Piracetam group [Table/Fig-3]. Similarly in MWM model test group cow ghee and butter group did not show any change in escape latency time in seconds during the study [Table/Fig-4]. To the best of our knowledge there are no studies available which correlate the impact of cow ghee and butter alone on dementia. Studies are available where cow ghee was used as vehicle/medium with other herbal drugs having memory enhancing action. In study conducted on Kushmandadi Ghrita [20] and Panchagavyaghrita [21] where cow ghee was used as vehicle, no nootropic activity was observed with cow ghee group alone, using EPM and MWM animal experimental models. Many Ayurvedic preparations having action on CNS are in Ghrutaform. In such preparation Cow Ghee is used as vehicle due to its lipoidal nature and it can easily cross blood brain barrier (BBB) [22].
The importance of cholesterol for brain function is attested by the fact that brain itself has >2% cholesterol by weight. However, the mechanism by which cholesterol affects memory is unknown [23]. Cholesterol is crucial for synapse generation and formation of synaptic vesicles [24]. Additionally, cholesterol is considered to be essential for remodeling neuronal membranes and growing new terminals, either during synaptic plasticity or in response to a neurodegenerative insult [25]. Manipulations of cholesterol in animals have shown a number of different relationships between cholesterol and memory.
Thus, dietary fatty acids may, under certain conditions, induce changes in neurophysiological, cognitive and other behavioural variables. There are many controversies about the effects of cholesterol on learning and memory, which pointed to the beneficial [23] or worsening or no effects of cholesterol [26,27]. It is suggested that high intake of saturated fat diet may decrease memory and learning ability by changing the brain fatty acid composition. It has been reported that hypercholesterolemia increases the levels of reactive oxygen species which facilitates the development of the neurodegenerative disease [28]. Decreasing cholesterol in frontal cortex of rats by a 1:4 mixture of a-linolenic and linoleic acid improved learning and memory for tasks such as the water maze [29]. Feeding mice a 2% cholesterol diet for eight weeks may result in deficits in working memory in the water Maze but not always [30]. Dietary cholesterol can influence a diverse number of learning tasks from water Maze to eyelid and fear conditioning Even though cholesterol added to the diet does not cross the BBB.
The traditional texts mentioned that cow ghee is a Medhya Rasayana, beneficial for mental alertness and memory in adults as well in children [4,5]. When we look into chemical composition of cow ghee and butter we found that though both cow ghee and butter have same amount of saturated fatty acid, monounsaturated, polyunsaturated fatty acids but they differ in type of fatty acid composition [31,32]. Milk butter is rich in cholesterol, myristic (14:0), palmitic (16:0), and lauricacid (12:0) that increases concentrations of LDL-c [33], whereas cow ghee contain more amount of Polyunsaturated Fatty Acids (PUFA), Omega 3 fatty acid and Docosahexaenoic acid (DHA), linolenic acid. DHA had been found higher in retina, cerebral cortex [11]. There are many references available which suggest beneficial role of omega 3 fatty acid and DHA, and PUFA in dementia. This supports the claim of Ayurvedic literature of usefulness/health benefit of Cow Ghee on cognition and lipid profile.
The reasons for not getting the significant results with cow ghee in present study was that dose of cow ghee (5% of diet) is not sufficient to provide Omega 3 or DHA that is required to increase cognition [34,35]. Dose should be given for longer period to get the effect or it should have been given in early stages of life as mentioned in Ayurvedic literature before brain development and for nerve growth.
The major concern for giving cow ghee for longer period with high dose is its effect on weight and lipid profile due to its high content of saturated fatty acid.
Effect of Cow Ghee and Butter on weight
When we assessed change in weight of rats after treatment with cow ghee and butter surprisingly reduction in weight was observed in piracetam and cow ghee group [Table/Fig-1]. Piracetam is known nootropic agent. From animal biochemistry it is known that nootropics enhance brain metabolism by stimulation of oxidative catabolism, increase of ATP-turnover and cAMP levels and enhancement of phospholipid-metabolism and protein biosynthesis. Piracetam have impact on the hippocampal release of acetylcholine and on the dopaminergic turnover, too [36]. This increase in central activity may be responsible for increasing activity and reduction in weight. This hypothesis should be studied further. Similar finding of reduction of weight with nootropics was reported by Gellisse [37], in patients.
In cow ghee group also reduction in weight was observed [Table/Fig-1,2]. This reduction in weight may be related to medicinal value of Cow Ghee which aids in fast absorption and digestion of food. Ghee stimulates the secretion of stomach acids to aid in digestion, while other fats and oils, can slow down the body’s digestive process and sit heavy in the stomach.
Other studies have also reported hyperactivity and irritability in rats fed with cow ghee [38]. This hyperactivity or CNS stimulation may be the other reason responsible for reduction in weight similar to effect of piracetam. Other reasons mentioned for weight reductions were interference with the absorption of essential nutrients from the gastrointestinal tract, increase in the metabolic rate unlinking it from energy formation mechanism (e.g., uncoupling of oxidative phosphorylation) and stimulation of the satiety center in the hypothalamus [38]. A moderate to significant increase in the fecal fat content was observed in all the ghee fed groups in same study.
Effect of Cow Ghee and butter on Lipid profile
Our findings with regard to serum lipid profile [Table/Fig-5,6] with ghee feeding were in accordance with the findings of Othman and Sharma et al., who observed that dietary ghee significantly increased the serum triglyceride levels [39,40]. However, these findings were in contrast to Kumar and Kathirvelan; and Kumar, who reported a significant decrease in plasma triglycerides in ghee-fed animals [41,42].
In the present study we observed that increase in TG and VLDL in Cow Ghee fed group was comparatively less than butter group [Table/Fig-5].
Increase in lipid level was generally observed when ghee or butter was given for more than four weeks [38–41]. We have given test drugs only for 21 days as primary aim of our study is to observe its effect on memory. This less duration of dosing may not be sufficient to cause beneficial effect. Increase in serum lipid profile in cow ghee and butter fed groups corresponds to its high content of saturated fats. Though butter and ghee’s chemical composition is nearly same they differ in type of fatty acid composition [31,32,40,43]. That is the reason increase in TG and VLDL in cow ghee group was less compared to butter group.
Myristic acid is the major contributor to the cholesterol raising effect of saturated fatty acids and that palmitic acid may be neutral, just like stearic and oleic acid. Myristic acid is mainly present in butter [33]. Palmitic acid and oleic acid is predominant fatty acid in both ghee and butter. However Ghee contain more amount of Polyunsaturated Fatty Acids (PUFA) (10.6 vs 4.1) and linolenic acid (0.4 Vs 0). Ratio of Omega6/Omega3m is 6.7 with ghee and 0.5 with butter [32].
PUFA are used to esterify cholesterol and excretion of it. Thus it will reduce cholesterol. The decrease in plasma triglycerides could be attributed to the anti-atherogenic effect of Conjugated Linoleic Acid (CLA) present in milk fat [42]. CLA as low as 0.05% level is found to reduce total triglycerides by 28%. The mechanism by which milk fat attenuates dietary hypercholesterolemia is not clear though CLA conjugate could be one of the contributing factors.
Ghee is a good source of short chain saturated fatty acids which are easier to digest [44]. Ghee also increases the excretion of dietary cholesterol and bile acids from gastrointestinal tract. This might be the other reason for less increase of serum cholesterol and LDL cholesterol levels in ghee group rats [45] than butter.
HDL is inversely related to the incidence of cardiovascular disease. In the present study HDL levels remained almost unchanged up to day 21 in both groups. The findings of the study are in contrast with Kathirvelan who reported a increase in HDL as a result of CLA enriched ghee feeding vs soybean oil control [42].
Ghee in spite of being a rich source of cholesterol and saturated fatty acids is considered good for the heart. One theory suggests that its lipid peroxidation that causes fat to become atherogenic (plaque forming) and animal saturated fat is resistant to the oxidation process and hence cannot cause the formation of plaque. On the contrary vegetable Polyunsaturated Fatty Acids (PUFA) are readily oxidized and PUFA-cholesterol esters are implicated in the process of plaque formation [46]. Another theory suggests that Ghee is rich in Antioxidants including Vitamin A, Vitamin E and carotenoids which may be helpful in preventing lipid peroxidation [7].
Limitation
As mentioned above we had not given test drugs for longer duration. We had used dose of cow ghee which was 5% of total calories, nootropic activity and change in lipid profile should be seen using other doses (2%, 10% etc). We had not measured consumption of feed or pellet; it should be done to see whether there is change in appetite or food consumption. As reported previously there were significant and noteworthy variations in the serum lipid profile of albino rats of varied ages and sexes. The mean TC, TG, VLDL and LDL of the male rats were significantly higher than those of the females. We had not considered this fact while grouping animals.
Conclusion
We cannot conclude beneficial effect of Cow Ghee and butter on cognition; and lipid profile from present study. Effect of cowghee on weight and serum lipid profile was better than butter. DHA along with fat-soluble vitamins, anti-oxidants, and conjugated linoleic acid (CLA), omega 3 acid could be responsible for health benefits of ghrita.
Results are given as mean ± SEM of six animals in each group.
Control group is compared with rest of the treated groups by one-way ANNOVA followed by Dunnett’s test. Significance at *p < 0.05; **p < 0.01; ***p < 0.001
Results are given as mean ± SEM of six animals in each group.
Control group is compared with rest of the treated groups by one-way ANNOVA followed by Dunnett’s test. Significance at *p < 0.05; **p < 0.01; ***p < 0.001
Results are given as mean ± SEM of six animals in each group.
Control group is compared with rest of the treated groups by one-way ANNOVA followed by Dunnett’s test. Significance at *p < 0.05; **p< 0.01; ***p < 0.001
Results are given as mean ± SEM of six animals in each group.
Control group is compared with rest of the treated groups by one-way ANNOVA followed by Dunnett’s test. Significance at *p< 0.05; **p < 0.01; ***p< 0.001