Dental caries has been traditionally described as a multifactorial disease that involves the interaction of various factors like host, agent, substrate and time. Landmark studies have established the fact that Streptococcus mutans (S. mutans) are the primary etiologic agent of dental caries. Hence, S. mutans plays a significant role in the development of dental caries [1]. One of the most powerful weapons that have been proved to control and prevent dental caries in the last 50yrs is Fluoride [2]. Various modalities i.e., water fluoridation, fluoride dentifrices, mouth rinses, varnishes and gels etc., have played a significant role in decline of dental caries. Although many vehicles have been suggested, fluoride mouth rinse is an effective adjunct to mechanical cleaning [3]. Triclosan is currently one of the most researched antimicrobial agents of the oral cavity and its use in preventive dentistry by incorporating it into various restorative materials and mouth washes has become well known because of its anti-bacterial qualities [4].
Much of the research on the effects of fluoride mouth rinse has been focused on the interaction between fluoride and dental hard tissues, while little attention has been paid to the effects of fluoride mouth rinses on the bacteria in the saliva. With various brands of mouthwashes available in the market this study was conducted to assess and compare the clinical efficacy of the four fluoride mouth rinses on the salivary levels of S. mutans and also to compare the efficacy of Triclosan containing and non Triclosan containing fluoride mouth washes by estimating the count of S. mutans in the saliva collected.
Materials and Methods
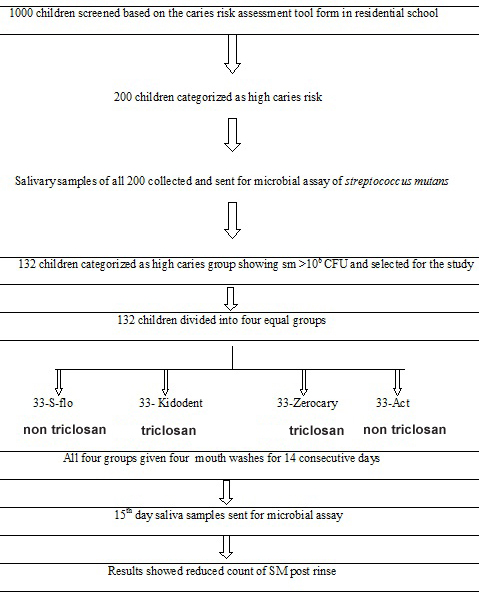
A randomized, controlled, double blind study was designed to find out the clinical efficacy of daily mouth rinsing with four fluoride mouth rinses, for 14 days, the study was done at Panineeya Mahavidhyalaya Institute of Dental Sciences (PMVIDS) in collaboration with Nizam Institute of Medical Sciences (NIMS) Department of Microbiology, Hyderabad, India. Since the study was a clinical trial spread over a period of 14 consecutive days the permission of the parents and school authorities was obtained [Table/Fig-1]. The ingredients of four mouth was shown in [Table/Fig-2].
Flow chart of the methodology.
The ingredients of four mouthwashes.
| Mouth wash | Components |
|---|
| S flo mouth wash | a. | Sodium Fluoride 0.2% |
| b. | Ponceau 4R & Erythrosine (coloring agents) |
| Kidodent mouth wash | a. | Sodium Fluoride 0.05% |
| b. | Xylitol 5% |
| c. | Triclosan 0.03% |
| d. | Brilliant blue FCF (coloring agent) |
| Act mouth wash | | Active ingredient: Sodium Fluoride 0.05%Inactive ingredients |
| 1. | Benzyl alcohol |
| 2. | Calcium disodium EDTA |
| 3. | Disodium phosphate |
| 4. | Sodium phosphate |
| 5. | Sodium benzoate |
| 6. | Sodium saccharin |
| 7. | Sorbitol |
| Zerocary mouth wash | a. | Sodium Fluoride 0.2% |
| b. | Triclosan 0.3% |
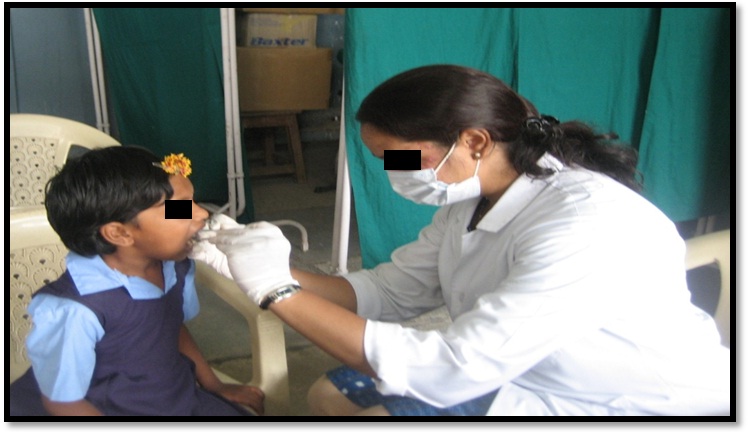
One thousand children (strength of the school included 1000 of the respective ages of 6-14 hence, all were screened) in the age group of 6-14 years were examined in local residential schools [Table/Fig-3]. Caries risk assessment was done based on Caries Risk Assessment Tool given by AAPD Guidelines 2011[Table/Fig-4]. In the caries risk tool form the biological and protective factors were filled by the investigator after interviewing each child. Clinical examination of each child’s oral cavity was done by the investigator.
Examination of the child.
Caries Risk Assessment Form (Age>6).
| Patient Name: Score:Birth Date: Date:Age: |
| Factors | High Risk | Moderate Risk | Protective |
| Biological |
| Patient is of low socioeconomic status | Yes | | |
| Patient has >3 between meal sugar containing snacks/ beverages per day | Yes | | |
| Patient has special health care needs | | Yes | |
| Patient is a recent immigrant | | Yes | |
| Protective |
| Patient receives optimally fluoridated drinking water | | | |
| Patient brushes teeth daily with fluoridated toothpaste | | | Yes |
| Patient receives topical fluoride from health professional | | | Yes |
| Additional home measures (e.g.: xylitol, MI paste, antimicrobial) | | | Yes |
| Patient has dental home/regular dental care | | | Yes |
| Clinical findings |
| Patient has ≥ 1 interproximal lesion | Yes | | |
| Patient has active white spot lesions or enamel defects | Yes | | |
| Patient has low salivary flow | Yes | | |
| Patient has defective restorations | | Yes | |
| Patient wearing an intraoral appliance | | Yes | |
Inclusion criteria involved written informed consent from the parents/guardian, good general health of children with pediatrician’s opinion, children not under any antibiotic coverage for the past three weeks, children not exposed to any fluorides for the past three weeks. Exclusion criteria were marked intraoral soft tissue pathology, medically compromised patients and children with restored teeth and children undergoing any orthodontic treatment or wearing any orthodontic appliances.
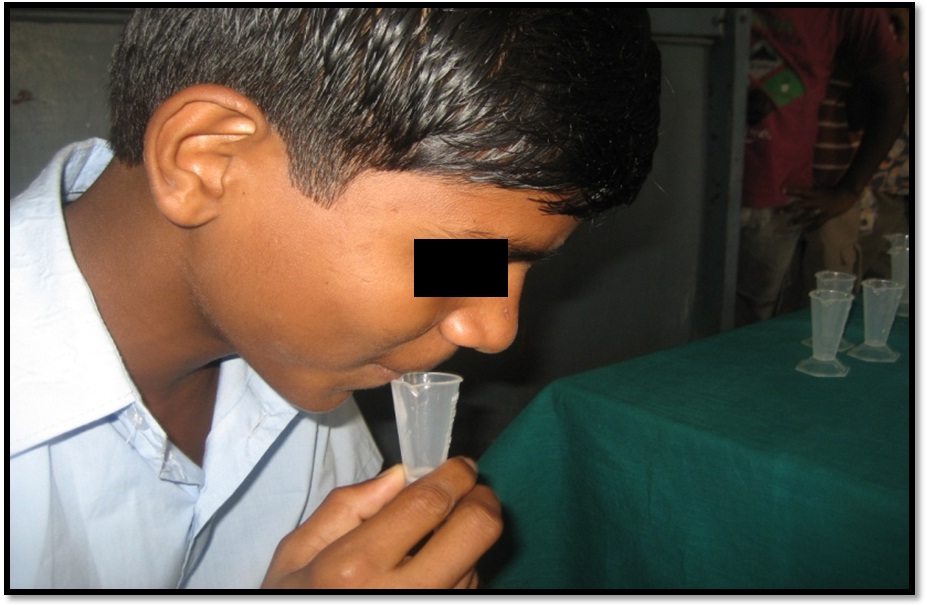
Out of the one thousand children screened based on the inclusion and exclusion criteria and using the caries risk assessment tool form, 200 children were categorized as high caries risk group. The children were given a non-fluoridated tooth paste for all the 14 days and were instructed to brush twice daily. The children were instructed to rinse their mouth thoroughly with water prior to the collection of saliva [Table/Fig-5]. The children were given 1.5g of paraffin wax and asked to chew. For every one minute, the children were asked to spit the saliva into the funnel without swallowing. After discarding the first two minute collection, the next three minute collection was saved for measuring the saliva flow rate. All the children who had a flow rate of less than 1ml were categorized under high caries risk group. Their saliva samples were collected and sent to estimate the pre rinse S. mutans count [Table/Fig-5].
Collection of saliva sample.
Out of 200 samples 132 saliva samples showed 106 CFU of S. mutans and were finally selected for the study [Table/Fig-6]. The study was double blinded. There were two investigators in the procedure. The first investigator was the examiner and the second investigator was one of the teaching staff. The second investigator performed the blinding of the study groups. The following methodology was followed. One hundred and thirty two dark amber colored bottles were selected and numbered 1-132. These bottles were now jumbled and randomly divided into four groups using a lottery method. Each group consisted of 33 bottles.
Bacterial colonies seen in MSB agar plate.
The four mouth rinses Act & S-flo (nontriclosan containing), kidodent and zerocary (triclosan containing) to be evaluated were assigned to each group respectively. The first examiner was not only unaware of the mouth rinse assigned to each group but also about the bottle numbered in each group. The selected 132children were assigned serial numbers from 1-132. The corresponding bottle numbers were allotted to the children with same serial number. E.g.: Bottle number 1 was given to child with serial no.1, so on so forth till 132.
The investigator himself demonstrated and trained the subjects regarding proper method of mouth rinsing. Daily attendance was marked for all the 132 children every day by the investigator. A graduated plastic jar was used to measure the quantity of mouth rinse to be dispensed every day. Only 10ml of the mouth rinse from the respective bottles was measured and dispensed to the subjects by the person who was unaware of the study. The children were asked to perform the mouth rinsing thoroughly for one minute and spit out the solution. After which the children were instructed not to eat, drink or wash their mouth for the next half an hour. The said procedure was repeated for a period of 14 consecutive days.
On the 15th day the stimulated midmorning saliva samples were collected and processed to assess the number of CFU of S. mutans and the results were obtained [Table/Fig-7]. The study was now unblinded and the entire data was sent for statical analysis using Paired t test while comparing the mean CFU counts of S.mutans among four different groups at 14 day interval and t- test was used for inter group comparison of the four different groups.
Reduced colony forming counts post rinsing after 14days seen on MSB agar plate.
Results
The results showed that at baseline there was no significant difference between the groups with respect to the number of S. mutans. After using the mouth washes for 14 consecutive days there was reduction in the bacterial counts. [Table/Fig-8]. When inter group comparison was made between all the four groups using a chi-square test Group D showed highest percentage reduction followed by Group A,B and C [Table/Fig-9,10]. When percentage reduction of CFU counts seen in the test groups, at ≤ 25% there were no subjects categorized under this group among all the four groups. At 25%-50% of CFU count reduction, Group A showed 18 subjects with a reduction of (54.54%), Group B showed 15 subjects with a reduction of (45.45%), Group C showed 18 subjects with a reduction of (54.54%) and Group D showed 13 subjects with a reduction of (39.39%). At 50%-75% of CFU count reduction, Group A showed five subjects with a reduction of (15.15%), Group B showed seven subjects with a reduction of (21.21%), Group C showed eight subjects with a reduction of (24%) and Group D showed seven subjects with a reduction of (21.21%). At 75%-100% of CFU count reduction, Group A showed 10 subjects with a reduction of (30.30%), Group B showed 11 subjects with a reduction of (33.33%), Group C showed 8 subjects with a reduction of (24%) and Group D showed 13 subjects with a reduction of (39.39%) [Table/Fig-10].
Comparison of mean CFU counts of S. mutans among four different groups at 14 day interval using paired t test.
| Group | | Mean | N | Std. Deviation | t | p-value |
|---|
| A | Pre | 8000000.00 | 33 | 4930770.731 | 1 .7 | <0.001 |
| Post | 3969697.27 | 33 | 3066806.246 |
| B | Pre | 7878787.88 | 33 | 5035856.281 | 1.4 | <0.001 |
| Post | 3727273.06 | 33 | 3105163.907 |
| C | Pre | 8969696.97 | 33 | 4544810.560 | 0.69 | <0.001 |
| Post | 4272727.52 | 33 | 2875246.653 |
| D | Pre | 7727272.73 | 33 | 5088422.688 | 2.9 | <0.001 |
| Post | 3363636.76 | 33 | 3219259.795 |
p = <0.001, Paired t-test
There was significant reduction in the CFU counts post rinsing for 14days among all the four groups.
Percentage reduction of CFU counts in test groups using chi-square test.
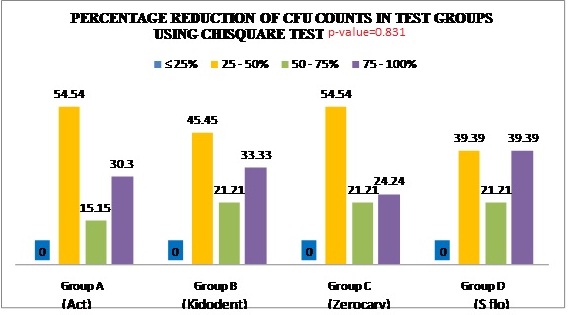
Percentage reduction of CFU counts in test groups.
| GROUP | ≤ 25% | 25-50% | 50-75% | 75-100% |
|---|
| A= Act (No.of subjects) | 0 | 18 | 5 | 10 |
| B= Kidodent (No.of subjects) | 0 | 15 | 7 | 11 |
| C= Zerocary (No.of subjects) | 0 | 18 | 7 | 8 |
| D= S-flo (No.of subjects) | 0 | 13 | 7 | 13 |
Group D (S-flo) showed greater % reduction followed by Group A, B & C.
Inter group comparison between Triclosan containing and non Triclosan groups using a t-test Triclosan group showed 52.5% reduction and non Triclosan group showed 53.3% reduction of cfu counts of SM [Table/Fig-11].
Comparison between triclosan containing and non containing groups.
| | Mean | N | Std. Deviation | p-value |
|---|
| (Act & Sflo)AD | pre | 7863636.36 | 66 | 4973425.885 | <0.001 |
| post | 3666667.02 | 66 | 3134587.442 |
| (Kidodent & Zerocary)BC | pre | 8424242.42 | 66 | 4791211.846 | <0.001 |
| post | 4000000.29 | 66 | 2981996.874 |
p = <0.001, t test
Pre and post rinse CFU count reduction seen between triclosan containing and non triclosan groups was not statically significant
Discussion
Good oral health is an integral component of good general health. Despite credible scientific advances and the fact that caries is preventable the disease continues to be a major public health problem [5]. Assessing patient’s risk of developing caries is a vital component of caries management. The assessment of all risk factors not only allows for a more accurate assessment of risk, but also identifies the etiologic factors responsible for the disease in a particular patient. Caries risk assessment form given by AAPD Guidelines 2011 has been used in order to categorize the children under high caries risk group from the total children screened in our study [6]. Mutans Streptococci are considered to be the most important group of bacteria initiating carious lesions. The number of salivary mutans in the oral cavity is correlated to the formation of new carious lesions and it is generally accepted that decreased number of S. mutans also reduces carious activity [7].
In the present study, saliva was used for estimation of bacterial count because saliva represents the oral load of microorganisms, as well as their average colonization in dentition [8]. According to the Caries risk assessment tool given by AAPD 2011 among the screened thousand children, 200 were categorized as high caries risk group. The saliva samples of these children were sent for microbiological tests, of which 132 children showed S. mutans counts in the range of 105-106 CFU/ml which correlated to the studies done by Klock B et al., [9], who reported that the subjects who had S. mutans count more than 106 are categorized as high caries risk group children. As it’s a proven fact by various studies that S. mutans is required for the initiation of dental caries hence, this organism is selected for our study [10]. Several media were proposed to quantify the level of S. mutans infection, among which Mitis Salivarius Agar (MSA) is the most commonly used media to isolate S. mutans thus, this selective media was used in our study [11].
The prevention of dental caries in children and adolescents is generally regarded as a priority for dental services and considered more cost-effective than its treatment. Treating the oral infections by decreasing the number of cariogenic bacteria and establishing a favorable environment to promote remineralisation of tooth structure over time will stop the carious process [12]. Fluoride therapy has been the centerpiece of caries preventive strategies.
The effectiveness of fluoride mouth rinses is primarily due to fluoride’s ability to enhance the process of remineralization, inhibit glucose transport, carbohydrate storage, extra cellular polysaccharide formation and acid production by oral streptococci [13]. In this context, many other chemical anti-plaque agents in various formulations have also been tried for improving oral health. One such noteworthy anti-plaque agent which gained popularity in recent times is Triclosan [14]. Despite being used in all these products it is still facing many controversies. According to the Federal Dental Association (FDA) in 2011 Triclosan showed no extra benefit to health. Animal studies have shown that Triclosan alters hormonal regulation and other studies on bacteria have raised the possibility that the use of Triclosan leads to bacterial resistance. However, an Expert Panel review concluded in 2000 that there was no evidence of resistant opportunistic or pathogenic microorganisms developing to Triclosan [15].
In the present study an attempt is made to assess the antimicrobial efficacy of four different fluoride mouth rinses in 6-12 year old school going high caries risk children. Among the 200 high caries risk children selected based on the caries risk assessment tool, 132 children showed >106 CFU counts. The 132 children were included in the study who were further divided into four groups including 33 children in each group.
The results showed that at baseline there was no significant difference between the groups with respect to the number of S. mutans. After 14 days of daily mouth rinsing with respective mouth washes the reduction in the S. mutans counts was observed between all the four groups [Table/Fig-8]. Our results are in correlation with the results of Kulkarni.V.V [16], Akihiro et al., [10], who in their studies concluded that by using NaF mouth rinse a significant reduction in the CFU of S. mutans was observed.
When the effectiveness of each fluoride mouth rinse group was evaluated Group D (S-flo) showed a higher percentage reduction which was followed by Group B (Kidodent), Group A (Act) and lastly Group C (Zerocary) [Table/Fig-9].
Group D containing S-flo mouthwash showed a higher percentage of CFU count reduction; this can be explained by the fact that S-flo has 0.2% of sodium fluoride (NaF) concentration. Studies have also proved that the higher the concentration of NaF greater is the reduction of CFU of streptococci [17].
Following Group D (S-flo) is Group A (Act mouth wash) and B (Kidodent) in reducing the CFU of S. mutans. This can be explained by the fact that both the mouthwashes had a same concentration of NaF of 0.05%. In addition these two fluoride mouth rinses also have xylitol as a common ingredient. In Act it is added as an inactive ingredient whose action is as an artificial sweetener. In Kidodent it is added at a 5% concentration as an active ingredient. According to the results of this study when Act and Kidodent are compared both have shown equal percentage reduction. Therefore, addition of either xylitol or Triclosan has not given any beneficial effect against reduction of S. mutans. This can be attributed to the fact that clinical trials of xylitol effectiveness in the form of mouth rinse are contradictory and limited, and it is an accepted fact that the most suitable form of delivery vehicle for xylitol is proposed to be chewing gum. Kidodent also has another key ingredient which is 0.03% Triclosan. According to our study this addition has not shown a significant effect in the S. mutans count, when compared with Act which only has 0.05% NaF as the main ingredient. On the contrary, another study done to evaluate the efficacy of sodium fluoride, chlorhexidine and Triclosan mouth rinses in reducing S. mutans count in saliva proved chlorhexidine followed by Triclosan are more effective when compared to sodium fluoride mouth rinses [16]. In another study significant reduction in plaque levels of S. mutans was observed with the use of xylitol, sodium fluoride and triclosan containing mouth wash [18]. In another study combination of fluoride and chlorexidine mouth wash showed significant decrease in salivary S. mutans count compared with fluoride alone, however the side effects of fluoride-chlorhexidine mouthwash were significantly more than the other two mouthwashes [19].
Lastly Group C (Zerocary mouthwash) which had a NaF concentration of 0.2% and 0.3% of Triclosan showed the least amount of percentage reduction in S. mutans counts between all the groups. Since it has 0.2% NaF and 0.3% Triclosan greater reduction in S. mutans counts was expected, but the results are contradictory the actual mechanism for this was not clear.
When inter group comparisons was made between Triclosan containing and non-Triclosan groups almost same percentage reduction of CFU counts was noted [Table/Fig-11].
With the increase in concern regarding bacterial resistance to antimicrobials worldwide, addition of Triclosan in mouth washes for children may not be recommended. This is supported by our study in which Group D which didn’t contain triclosan showed greater % reduction followed by Group C, A and B with varying concentrations of fluoride and triclosan.
With the surfeit of mouth rinses available in the market this study was carried out to compare the clinical efficacy of four popularly prescribed fluoride mouth rinses on the salivary levels of S. mutans. With the increase in concern regarding bacterial resistance to antimicrobials worldwide, inadvertent addition of Triclosan in mouth washes for children may not be recommended. The present study shows that 0.2% NaF gives the best reduction of S. mutans when used as mouth wash in children with high caries risk.
Limitation
Limitations of the study include the continuous monitoring of the children on proper rinsing technique of the mouth wash. There is no scientific literature available regarding the comparison of different mouth washes containing both sodium fluoride and Triclosan. This is one of few studies done to evaluate and compare fluoride mouth washes containing both sodium fluoride and Triclosan. Further research is needed to study the mechanism of action of Triclosan and fluoride when added together for better clinical application.
Conclusion
The following conclusions were drawn from the study. The mean pre rinse CFU was significantly higher than post rinse CFU for all the study groups, suggesting that all the four mouth rinses were effective in decreasing the levels of Streptococcus mutans in the saliva. There was no significant difference in the mean percentage reduction of CFU among the four study groups (p = 0.75) but when compared among all the four. Group D (S flo) had high cfu reduction followed by Group C(Kidodent), Group A(Act) and finally Group B(Zerocary)and finally, both the Triclosan containing and non Triclosan groups showed no significant difference in amount of CFU count reduction.
p = <0.001, Paired t-test
There was significant reduction in the CFU counts post rinsing for 14days among all the four groups.
Group D (S-flo) showed greater % reduction followed by Group A, B & C.
p = <0.001, t test
Pre and post rinse CFU count reduction seen between triclosan containing and non triclosan groups was not statically significant