Imaging Findings in Dysgerminoma in a Case of 46 XY, Complete Gonadal Dysgenesis (Swyer syndrome)
Pratiksha Yadav1, Sanjay Khaladkar2, Aditi Gujrati3
1 Associate Professor, Department of Radio-diagnosis, Dr. D. Y. Patil Medical College, Hospital and Research Centre, Pimpri, Pune, Maharashtra, India.
2 Professor, Department of Radio-diagnosis, Dr. D. Y. Patil Medical College, Hospital and Research Centre, Pimpri, Pune, Maharashtra, India.
3 Resident, Department of Radio-diagnosis, Dr. D. Y. Patil Medical College, Hospital and Research Centre, Pimpri, Pune, Maharashtra, India.
NAME, ADDRESS, E-MAIL ID OF THE CORRESPONDING AUTHOR: Dr. Pratiksha Yadav, Associate Professor, Department of Radiodiagnosis, Dr. D.Y. Patil Medical College Pune-411017, Maharashtra, India.
E-mail: yadavpratiksha@hotmail.com
A 46 XY pure gonadal dysgenesis also known as Swyer syndrome. These patients are phenotypic females with normal female external genitalia and absent testicular tissue. The patients with swyer syndrome have streak gonads and increased risk of dysgerminoma and gonadoblastoma. We present a case of dysgerminoma in dysgenetic gonads of swyer syndrome. A 23-year-old female had come with complaints of primary amenorrhea, pelvic mass and abdominal pain. Clinical findings, pathology investigation and imaging findings revealed swyer syndrome. On MRI it showed a large lobulated mass in the pelvis. Mass was excised and dysgerminoma was given on the histopathology.
Amenorrhea, Gonadoblastoma, Sexual dysgenesis
Case Report
A 23-year-old female patient visited the Department of Radiology with complaints of lower abdominal pain and primary amenorrhea. No history of fever, burning micturition and no bowel complaints was present. No significant family history was present. On examination there was under-development of breast tissue. Only breast bud was formed, no glandular tissue (Tanner II). There were absent axillary hair however sparse pubic hair was present. Female external genitalia were normal, normal appearing vulva and vagina was seen. A large, firm pelvic mass was palpable which was of approximately 10-12 cm in size. Ultrasonography was advised which revealed a large heterogeneous pelvic mass which showed mixed echotexture [Table/Fig-1]. Uterus and ovaries were not visualized separately from the mass. On Colour Doppler there was significant flow seen in the mass with neovascularization [Table/Fig-2]. Contrast enhanced CT scan of the abdomen and pelvis was done which revealed a large lobulated mass occupying the pelvis posterior to urinary bladder and anterior to rectum [Table/Fig-3a-c]. It measured approximately 13.4cm (craniocaudal) X 8.9cm (antero-posterior) x 12.4cm (Transverse) dimensions. Superiorly it extended till umbilical level. It showed heterogeneous soft tissue density (ranging from 25-48 HU). It showed heterogeneous enhancement on post contrast study in venous and delayed phase and showed neovascularity in arterial phase [Table/Fig-3a]. Few ill-defined hypodense non-enhancing areas were also seen suggestive of necrosis/cystic degeneration [Table/Fig-3b]. Few tiny specks of calcification were also seen at places. The mass compressed the posterior wall of urinary bladder anteriorly and rectum was pushed posteriorly [Table/Fig-3c]. Intervening fat plane between mass and bladder as well as rectum were well defined. Uterus and both ovaries are not visualized separately from the mass. No lymphadenopathy seen. Liver, kidneys and spleen were unremarkable. No evidence of metastasis seen. Mild free fluid was also seen in the pelvis. MRI was done for further evaluation which revealed a large heterogeneous, lobulated mass seen posterior to urinary bladder occupying the region of uterus [Table/Fig-4a] which measured approximately 11.4cm (CC) X 7.1cm (AP) X9.1cm (Trans). This mass lesion was predominantly hyperintense on STIR [Table/Fig-4b&c], heterogeneously mixed signal intensity on T2WI and isointense on T1WI [Table/Fig-4d]. Few septae were seen in the mass. This mass pushed urinary bladder anteriorly and rectum posteriorly, intervening plane between mass and bladder and mass and rectum was maintained. On post gadolinium MRI this mass showed moderate heterogeneous enhancement with enhancement of septae [Table/Fig-5a&b]. Both the ovaries and fallopian tubes were not visualized. Vagina appeared normal. Mild free fluid was seen in the right paracolic gutter and in the pelvis. Few small inguinal lymph nodes were seen bilaterally.
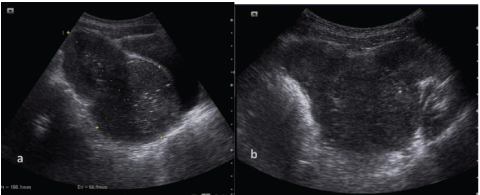
Pelvis ultrasonography image of the 23-year-old female showing a large, lobulated heterogeneous mass occupying the pelvis posterior to urinary bladder.
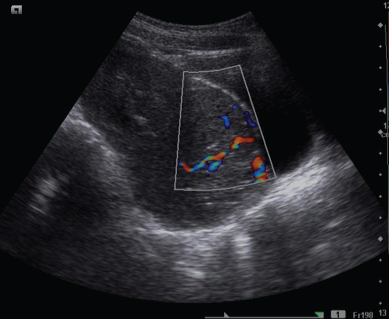
Colour Doppler of the lobulated heterogeneous mass pelvic mass showed increased vascularity.
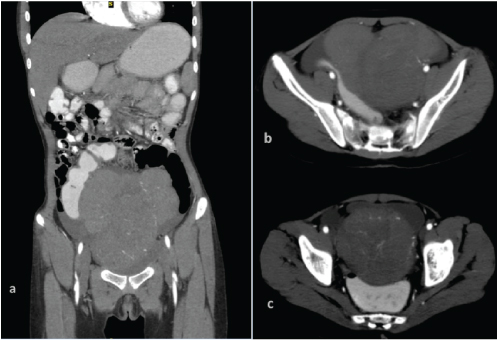
Contrast enhanced CT abdomen and pelvis of the patient showed a heterogenous lobulated mass occupying the region of the uterus.
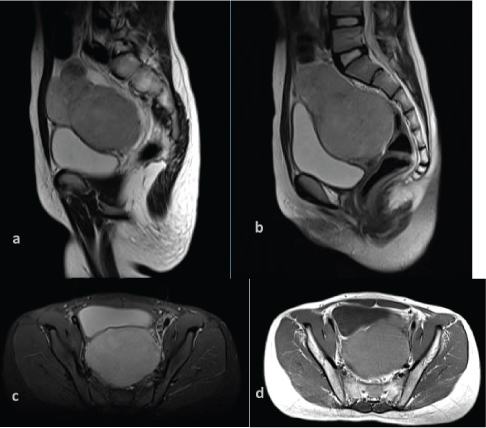
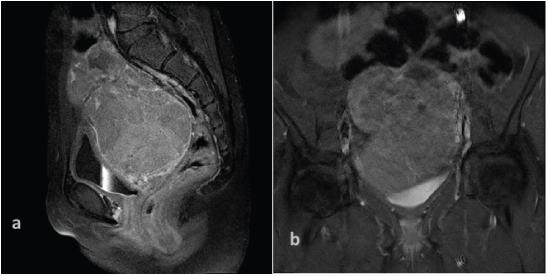
Magnetic resonance imaging of the pelvis showed a lobulated mass posterior to bladder and anterior to rectum. Sagittal T2WI showing mixed signal intensity (4a), Sagittal and axial STIR images showing mildly hyperintense signal (4b&c), Axial T1WI showing isointense signal intensity (4d).
Post gadolinium sagittal and coronal MRI images showed moderate heterogenous enhancement of the mass.
Blood investigations: DHEA-SULPHATE – done by CLIA method value – 329 μg/dl (normal range). Prolactin was 7.65 ng/ml (Lower side of normal menstruating females -2.8-29.2). Testosterone – 10ng/dl (adult male -241-827, adult female -14-76) was low. Alpha-fetoprotein (AFP) 0.65IU/ml (normal). Beta HCG – 61.4mIU/ml (increased –normal non prg females - <30mIU/ml, Males- <2.5mIU/ml).
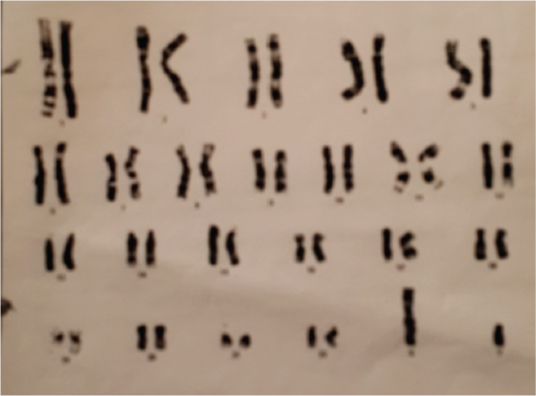
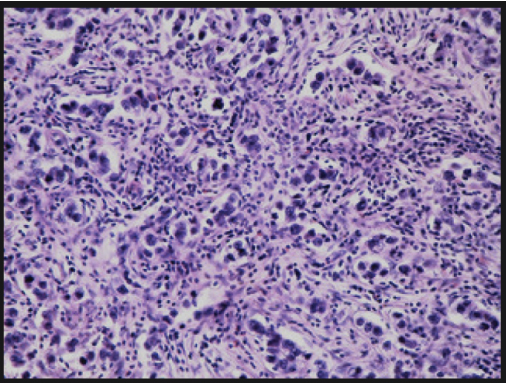
Chromosomal analysis (Karyotype) of blood was done; interpretation of the test was abnormal showed male karyotype [Table/Fig-6]. No numerical or structural chromosomal anomalies detected at 450-550 banding resolution consistent with Androgen Insensitivity syndrome (Testicular feminization). Exploratory laparotomy was done. A solid mass of approximately 15x12x10 cm seen, probably arised from right streak ovary, left sided fallopian tube and streak ovary was noted. The mass was excised and sent for histopathology examination. On histopathology it showed nest of neoplastic cells separated with fibrous tissue septae consistent with dysgerminoma [Table/Fig-7].
Chromosomal analysis (Karyotype) of blood showing male karyotype (46 XY). No numerical or structural chromosomal anomalies detected at 450-550 banding resolution consistent with Androgen Insensitivity syndrome (Testicular feminization).
Histopathology of the exiced mass showed nest of neoplastic cells separated with fibrous tissue septae consistent with dysgerminoma.
Mass showed multinodular, lobulated appearance, cut surface showed areas of necrosis and hemorrhages. Staging of the disease was stage II a (involvement of bilateral ovaries, fallopian tubes and uterus)
Discussion
A 46XY pure gonadal dysgenesis or Swyer syndrome is a rare disorder of sexual dysgenesis, described by Swyer in 1955 [1,2]. These patients are phenotypic females who have female external genitalia, normal mullerian structure and absent testicular tissue. These patients are diagnosed as delayed pubertal development. Patients with disorder in sexual differentiation have increased risk for developing genital malignancies. Dysgerminoma are rare female germ cell tumour histologically corresponding to seminoma [3]. These tumours account for 1% of all ovarian malignancies and around 50% of all ovarian germ cells malignancies [4].
Swyer syndrome is a pure gonadal dysgenesis associated with 46XY karyotype. These are phenotypic females who have primary amenorrhea, presence of female internal and external organ with no genital ambiguise. In these patients mesonephric ducts are in atrophy. Paramesonephric ducts (mullerian ducts) develop to uterus, fallopian tubes and superior part of vagina. Uterus is usually present in these patients and usually hypoplastic [5]. They show increased level of gonadotropins, low levels of estrogen and normal female levels of androgens [6]. The patients with swyer syndrome have streak gonads and increased risk of malignancies. Gonadoblastoma and dysgerminoma are the most frequent malignant genital tumours seen in swyer syndrome. Risk of malignancy in these patients is approximately 30% [7–9]. Bilateral gonadectomy is advised as soon as the diagnosis is made [4].
Swyer syndrome is the uncommon form of the gonadal dysgenesis as compare to Turner syndrome and androgen insensitivity syndrome. Incidence of Swyer syndrome is 1:100,000, incidence of Tuner syndrome is 1:2000 and incidence of androgen sensitivity syndrome is 1:20,000 – 64,000 [8,10–12].
Dysgerminoma is most common malignant germ cell tumour of the ovary and comprise about 1% of the ovarian malignancies. The tumour arises from the primordial germ cells and considered as ovarian counterpart of the seminoma of the testis, they are usually solid and multilobulated tumours. These tumours are hormonally inert and not associated with endocrine hormonal secretion though the syncytiotrophoblastic giant cells, which produce HCG are present in 5% of dysgerminomas and that can cause elevation of serum HCG level [13,14]. Approximately 65% of the dysgerminoma are stage I at the time of diagnosis (confined to one or both ovaries). About 85-90% of stage I tumours are confined to one ovary and 10-15 % are bilateral [15]. In stage II it involves one or both ovaries and spread to pelvic structures, fallopian tubes and/or uterus. In stage III when peritoneal implants are outside pelvis and stage IV when distant metastasis occur.
Conclusion
Swyer syndrome is very rare disorder of sexual dysgenesis with increased risk of malignancy. Accurate diagnosis is very important and early surgery is recommended to reduce the mortality and morbidity. CT scan and Magnetic resonance imaging are excellent modality to see the extent of tumour and accurate staging of the disease. MRI is very helpful in the diagnosis as it is helpful in the evaluation of pelvic mass and the status of ovaries.
[1]. Berek JS, Hacker NF, Clinical Gynecology 2004 Chapter 234th edition:514-18. [Google Scholar]
[2]. Speroff L, Feritz MA, Clinical Gynecologic Endocrinology and infertility 2005 7th edition:322-25.:348-49.:386-87. [Google Scholar]
[3]. Cheng L, Thomas A, Roth LM, Zheng W, Michael H, Karim FW, A novel biomarker for dysgerminoma of the ovaryAmer J Surg Pathol 2004 28(10):1341-46. [Google Scholar]
[4]. O’Neill JA, Grosfeld JL, Principles of Pediatric Surgery. 220 × 283 mm. Pp. 895. Illustrated 2004 New YorkMosby (Elsevier) [Google Scholar]
[5]. Dapunt O, Platzer W, Gleispach H, Millonig-Ganner B, Rudimentary uterus in testicular feminization (author’s transl)Wien Klin Wochenschr 1975 87(16):498-506. [Google Scholar]
[6]. Wang YHY, Li Q, Dai S, He A, Wang E, Dysgerminoma in a case of 46, XY pure gonadal dysgenesis (swyer syndrome): a case reportDiagn Pathol 2011 6:84Published online 2011 Sep 19 [Google Scholar]
[7]. Ben Romdhane K, Bessrour A, Ben Amor MS, Ben Ayed M, Pure gonadal dysgenesis with 46 XY karyotyping (Swyer’s syndrome) with gonadoblastoma, dysgerminoma and embryonal carcinomaBull Cancer 1988 75:263-69. [Google Scholar]
[8]. Vollrath D, Foote S, Hilton A, Page DC, The human Y chromosome Swyer GIM. Male Pseudohermaphroditism: A Hitherto Underscriebed FormBr Med 1995 2:709-12. [Google Scholar]
[9]. Coutin AS, Hamy A, Fondevilla M, Savigny B, Paineau J, Visset J, Pure 46XY gonadal dysgenesisJ Gynecol Obstet Biol Reprod (Paris) 1996 25:792-96. [Google Scholar]
[10]. Quigley CA, De Bellis A, Marschke KB, El-Awady MK, Wilson EM, Androgen receptor defects: historical, clinical and molecular perspectivesEndocr Rev 1995 16:271-321. [Google Scholar]
[11]. Bangsbøll S, Qvist I, Lebech PE, Lewinsky M, Testicular feminization syndrome and associated gonadal tumours in DenmarkActa Obstet Gynecol Scand 1992 71:63-66. [Google Scholar]
[12]. Gravholt CH, Clinical practice in Turner syndromeNat Clin Pract Endocrinol Metab 2005 1:41-52. [Google Scholar]
[13]. Song ES, Lee JP, Han JH, Dysgerminoma of the ovary with precocious puberty: a case reportGynecol Endocrinol 2007 23(1):34-37. [Google Scholar]
[14]. http://radiopaedia.org/articles/ovarian-dysgerminoma [Last Accessible 31st August 2016] [Google Scholar]
[15]. Casey AC, Bhodauria S, Shapter A, Nieberg R, Berek JS, Farias-Eisner R, Dysgerminoma: the role of conservative surgeryGynecol Oncol 1996 63(3):352-57. [Google Scholar]