Regeneration of the tissues lost due to periodontal diseases has long been the aim and ultimate goal of periodontal therapy [1]. Although conventional therapy such as scaling, root planning and gingival curettage are highly effective at repairing disease related defects and halting the progression of periodontitis, but they do relatively little to prompt the regeneration of the lost periodontal support structures [2]. Thus, more effective techniques that promote the natural ability of the body to regenerate its lost periodontal tissues i.e., formation of new functional tissues rather than to build new replacements of periodontium, need to be applied. The objective is to reconstitute the biologic complex of cementum, periodontal ligament and alveolar bone onto the root surface.
1) The use of bone grafts or bone substitutes.
2) Guided cell repopulation using barrier membranes regeneration.
3) Tissue engineering through the use of biomimetic agents.
These techniques have demonstrated clinical success in varying degree, in prompting periodontal regeneration in periodontal sites, but today the principle of Guided Tissue Regeneration (GTR) forms the basis for most regenerative periodontal procedures. One of the pre-requisite of a GTR membrane is that it should be stiff enough to resist collapse from the pressure of the overlying tissue [4]. For this reason, some of the membranes were modified by incorporation of Titanium reinforcements (Titanium reinforced ePTFE) the rigidity of which supports improved space provision and maintenance thus resulting in significantly greater Clinical Attachment Level (CAL) improvements than conventional GTR or access flaps [5]. In 2003, Wong introduced a new non-resorbable barrier membrane made of titanium called “Ultra-Ti® GTR membrane” [6].
Its advantages are that it is pure titanium membrane of homogenous structure and ultra-thin thickness (about 10 microns) with less surface roughness as compared to the other barrier membranes. It is biocompatible and non-immunological, readily pliable, moldable and adaptable because of its ultra- thin thickness. It has perfect adaptation and passivity and does not require fixation with screws or pins (unlike the thick titanium membrane and the Gore-Tex membrane). It doesn’t rebound and slip. It is non-space occupying, space maintaining membrane, leading to easy closure. Primary closure is not necessary with this membrane. The wound stays clean as its smooth surface makes it less susceptible to bacterial contamination than resorbable materials. Since it is radiolucent it, allows monitoring of bone formation. Few disadvantages being that it is a non-resorbable membrane, thus requires a second surgical procedure for removal. It is a non-porous membrane therefore it does not allow tissue integration, leading to pouch formation.
It was proposed to conduct a clinical case control study in Department of Periodontics, Goa Dental College and Hospital, Goa, India, using this membrane for the treatment of intraosseous, interproximal periodontal defects in comparison with open flap debridement judged over a period of nine months.
The current study aimed to study the amount of regeneration of the periodontium along with hard tissue fill in the interproximal bony defect using Ultra-Ti® membrane and to compare it with the bone fill achieved in control sites by open flap debridement alone.
Materials and Methods
This spilt-mouth, case control study was conducted in the year 2004-2005, after obtaining clearance from the ethical committee of Goa Dental College and Hospital, Goa, India, and included 17 patients (8 males and 9 females) between 25 years to 50 years of age, who were treated at the Department of Periodontics, for inflammatory periodontal disease. Out of these three did not come back for follow-up and two patients had their membranes displaced. Hence, they were not included in this study. Thus, the study included only 12 patients. All patients were informed about the treatment of the periodontal defects and were told to give their written consent.
Patient Selection: Patients in the age group of 25–50 years with good general health and without any history of systemic diseases or compromising medical conditions were selected. Intra-orally, presence of at least two radiographically detectable interproximal infrabony osseous defects in at least two teeth on the contralateral or opposite arch with a probing depth ≥5mm following initial therapy, depth of the intraosseous component of the defect ≥3mm as measured by bone sounding and radiographic means were selected and patients maintaining good oral hygiene prior to surgical therapy were included. Patients on any drug therapy, which may interfere with the healing of the tissues e.g., immunosuppressive drugs like corticosteroids, pregnant and lactating women, patients who underwent periodontal therapy in the past six months in the affected area, patients with habits like smoking or use of other tobacco products, teeth showing evidence of periapical infection and inability or unwillingness to give informed consent. All such patients were excluded from this study.
Clinical Study Design: Shown in flowchart [Table/Fig-2].
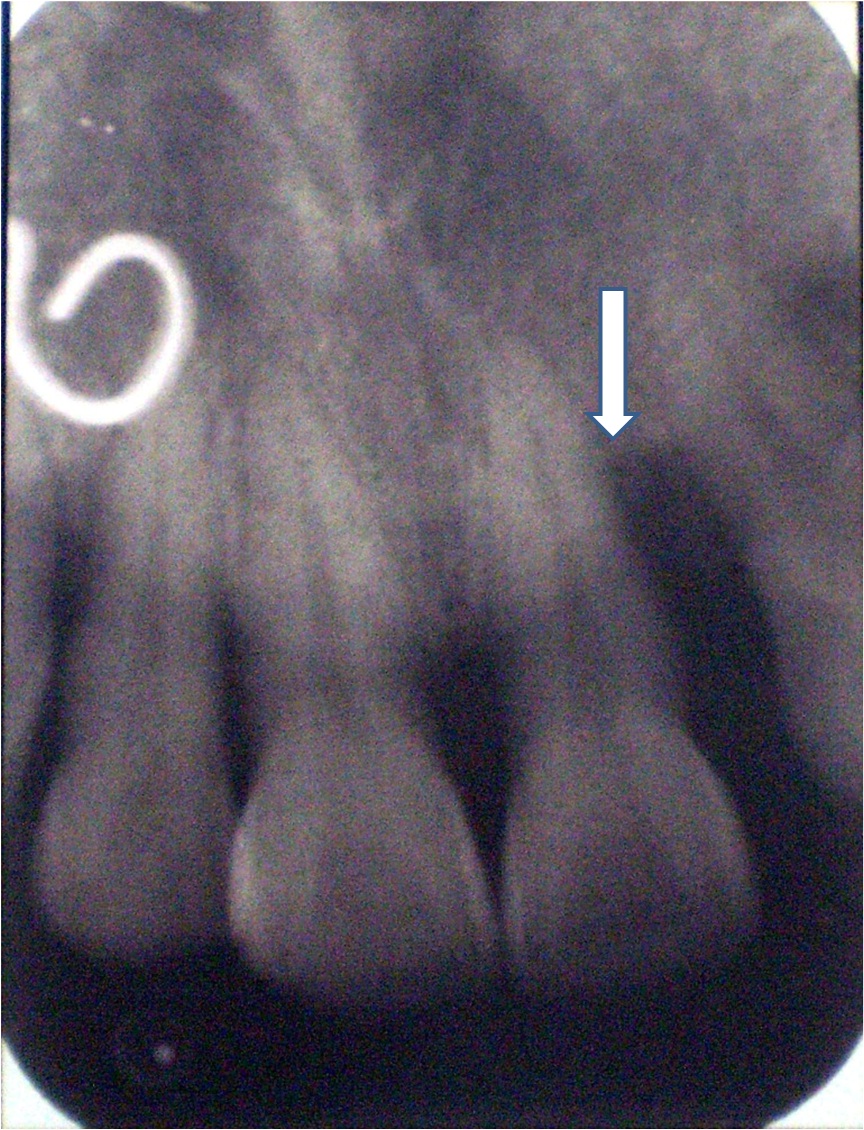
Radiographic Examination: An intra-oral periapical radiograph was taken of each selected site with the long cone (XCP Rinn, Dentsply, USA.) paralleling technique at baseline and 9 months after surgery [7].
Hard Tissue Measurements: These measurements were made at the time of surgery: -
Distance from the lower border of the stent to the alveolar crest (Stent-AC)
Distance from the lower border of the stent to the base of the defect (Stent-BD)
The intra-bony component was obtained by subtracting (Stent-BD) - (Stent-AC) i.e., the distance between the alveolar crest to the base of the alveolar defect.
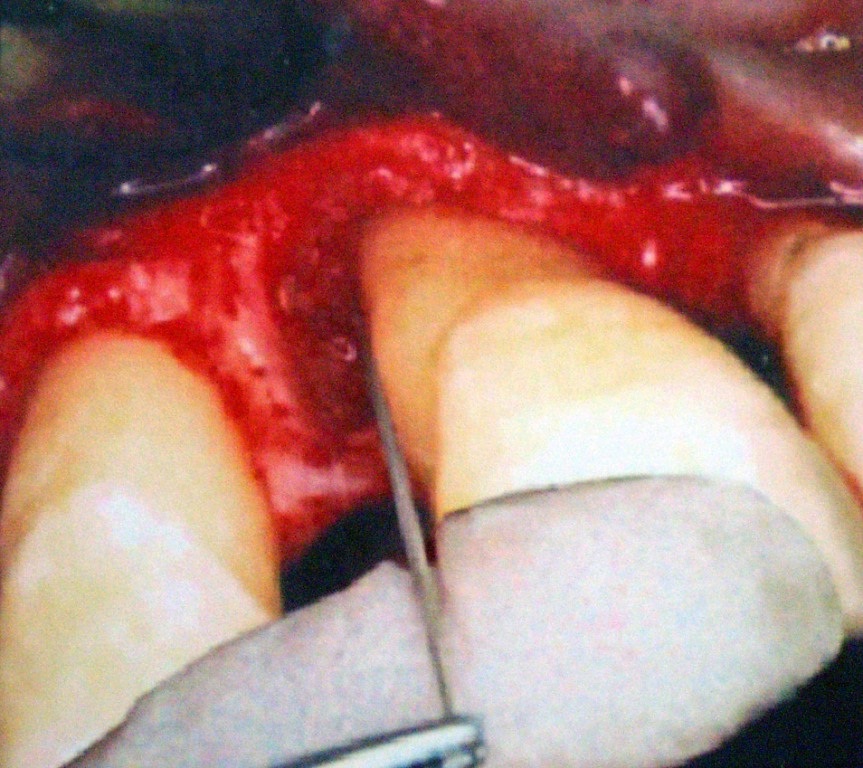
Procedure: Two weeks after completion of the baseline examination and thorough scaling and root planning, the infra-bony defects were assigned to either experimental or control sites. Following administration of local anesthesia, incision and debridement were carried out. One osseous defect (i.e., test site) was treated with Ultra-Ti® membrane after open flap debridement and the other osseous defect was treated only by open flap debridement. The flaps were sutured with interrupted sutures in order to get primary, tension-free wound closure. A non-eugenol periodontal pack was placed over the surgical sites.
Immediate post-operative examination was carried out on the 2nd, 5th, 7th, 14th and 21st day to check for post surgical pain, edema, swelling, infection, wound dehiscence and barrier exposure.
After 5-6 weeks post the surgical procedure, the membrane was carefully removed from the experimental site so as not to disturb the underlying new connective tissue formation. Oral hygiene was resumed by the patient. Recall appointments were made after one month, three months and six months. At each visit, oral hygiene instructions were reinforced and the surgical sites were professionally cleaned and irrigated with normal saline.
At the end of nine months post surgery, patients were evaluated clinically and radiographically. Clinical parameters were repeated. A re-entry procedure was performed for the test and control sites i.e., a mucoperiosteal flap was raised in the most atraumatic manner in the initial surgical site and the hard tissue measurements were repeated [Table/Fig-3,4,5,6,7,8,9 and 10].
Pre-operative probing depth.
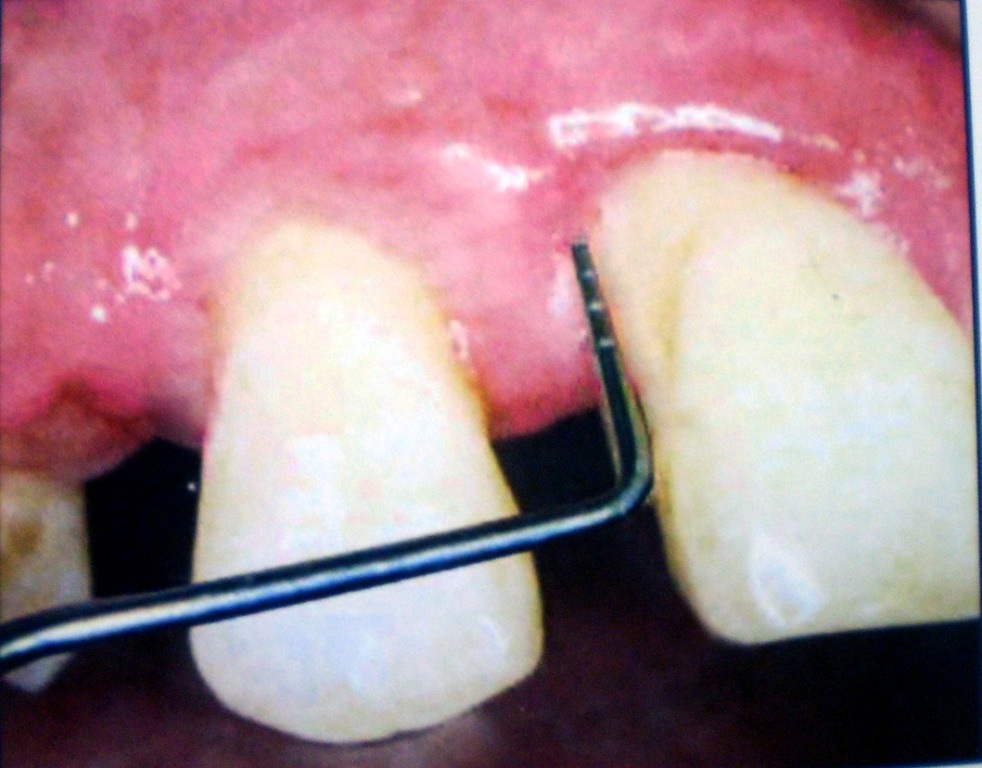
Pre-operative Relative attachment Level.
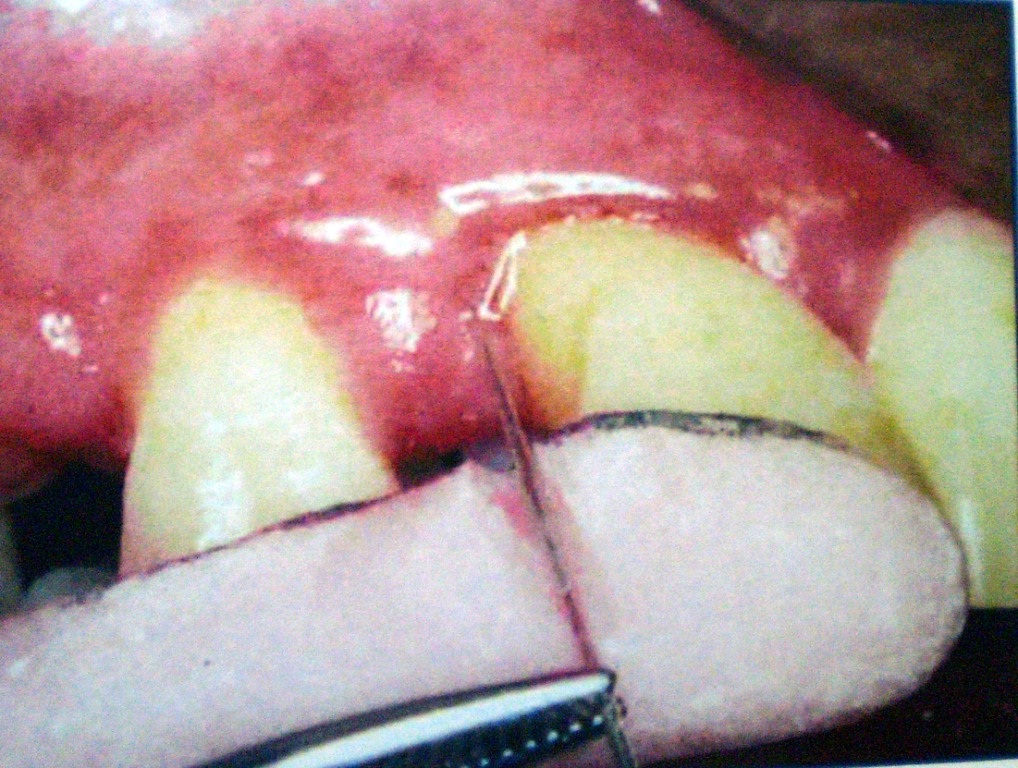
Pre-operative Depth of Defect.
Pre-operative Radiograph.
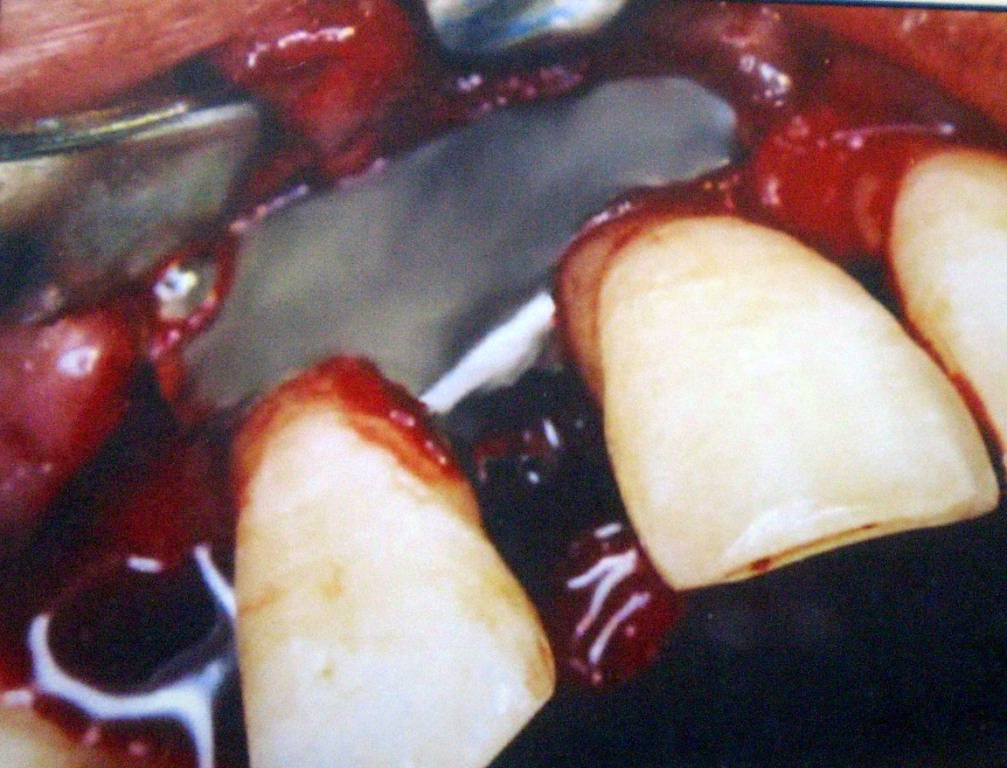
Placement of the Membrane.
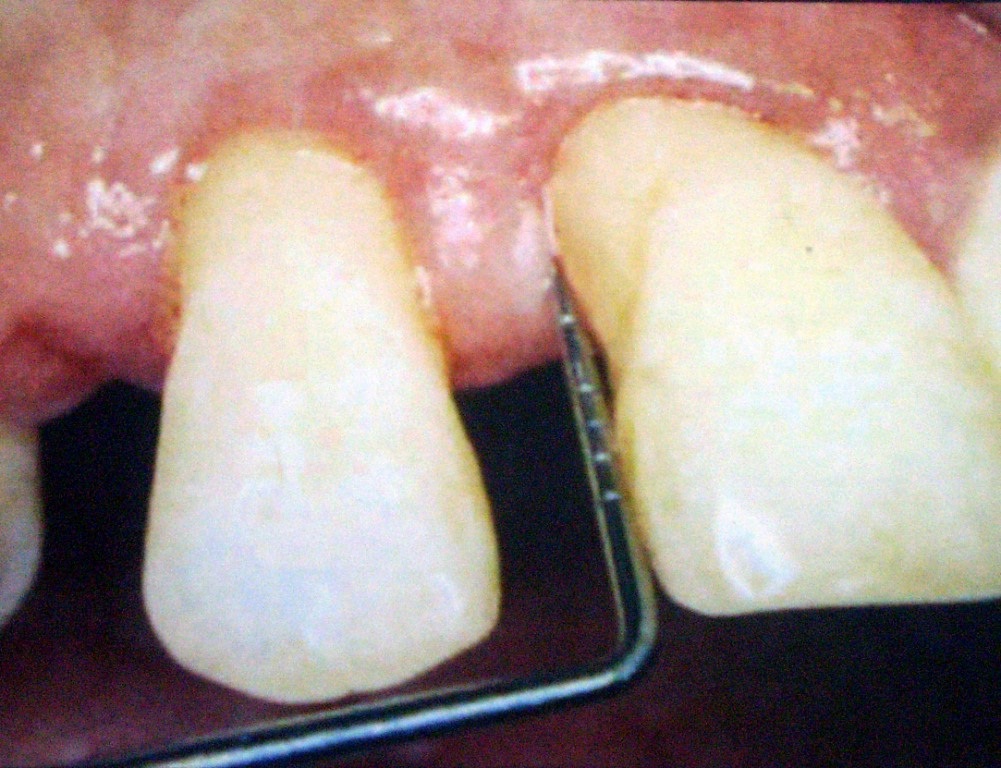
Post-operative probing depth.
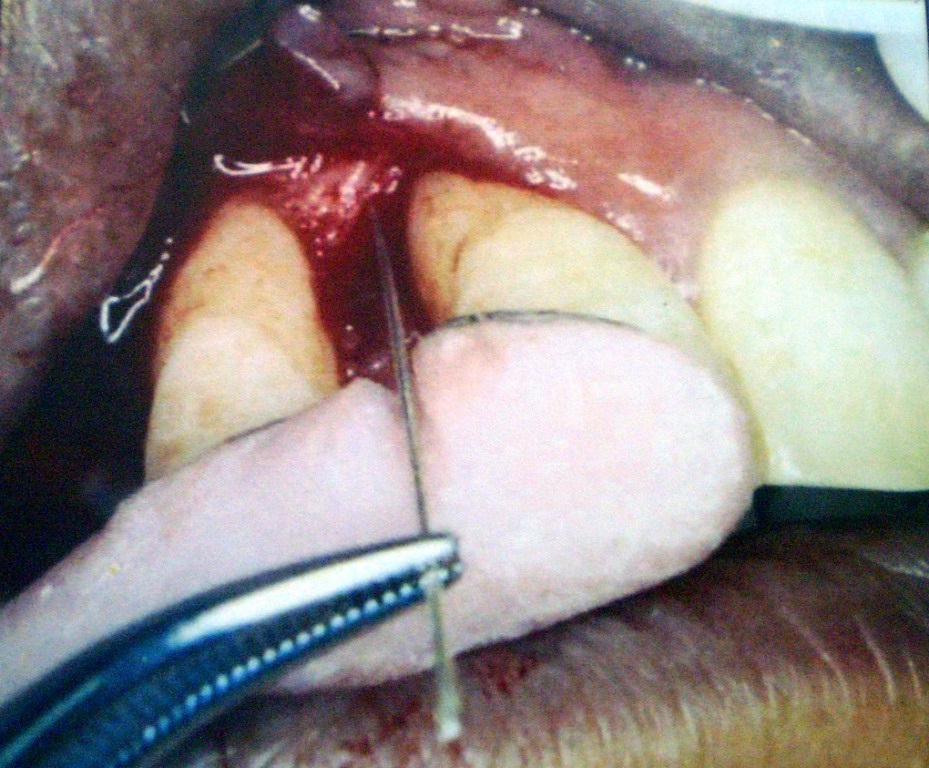
Post-operative depth of defect.
Post-operative Radiograph.
Results [Table/Fig-11,12]
Intragroup observations of values of test & control groups at baseline and nine months post-op.
| Intragroup Observations |
|---|
| Test Site | Control Site |
|---|
| Baseline | 9 months | Baseline | 9 months |
|---|
| Mean Plaque Index | 1.641 | 0.738 | 1.662 | 0.783 |
| p-value | 0.00001 (S) | 0.000002 (S) |
| Mean Gingival Index | 2.058 | 0.896 | 2.042 | 0.896 |
| p-value | 0 (S) | 0 (S) |
| Mean Probing Pocket Depth(mm) | 7.333 | 2.417 | 6.833 | 3 |
| p-value | 0 (S) | 0 (S) |
| Mean RAL gain (mm) | 12 | 7.625 | 11.167 | 7.75 |
| p-value | 0 (S) | 0 (S) |
| Mean Gingival Position (mm) | 4.667 | 5.208 | 4.333 | 4.75 |
| p-value | 0.003 (S) | 0.05 (S) |
| Mean Defect Depth Reduction (mm) | 5.583 | 2.5 | 4.667 | 4.25 |
| p-value | 0.00 (S) | 0.09 (NS) |
*Statistical Analysis done by Student’s paired t-test
Intergroup observations of values of test & control groups at baseline & 9 months post-op.
| Intergroup Observations |
|---|
| Test Site | Control Site |
|---|
| Mean Plaque Index | 0.904 | 0.878 |
| p-value | 0.816 (NS) |
| Mean Gingival Index | 1.079 | 1.146 |
| p-value | 0.6 (NS) |
| Mean Probing Pocket Depth (mm) | 4.917 | 3.833 |
| p-value | 0 (S) |
| Mean RAL Gain (mm) | 4.375 | 3.417 |
| p-value | 0.04 (S) |
| Mean Gingival Position (mm) | 0.541 | 0.417 |
| p-value | 0.67 (NS) |
| Mean Defect Depth Reduction (mm) | 3.083 | 0.417 |
| p-value | 0.00 (S) |
*Statistical Analysis done by Student’s paired t-test
Student’s paired t-test and unpaired t-test (SPSS software version 9) were used to analyze the results. It was observed that there was a significant decrease in mean plaque scores and gingival index from baseline to nine months post-operatively in both test and control sites (p<0.001).
Also a significant reduction in Probing Pocket Depth (PPD) between baseline and nine months post surgery for both test and control sites was seen with mean reduction of 4.917 ± 0.996mm and 3.83 ± 0.718 mm respectively (p<0.001). Also, there was a statistically significant difference found between the two groups, with the test site showing increased probing pocket reduction as compared to the control site (p<0.001).
The Relative Attachment Level (RAL) gain was seen to be higher in the test site (4.375 ± 1.189 mm) as compared to 3.417 ± 0.996 mm in control site (p<0.001).
The amount of gingival recession nine months post surgery for test and control sites were 0.541 ± 0.498 mm and 0.471 ± 0.669 mm. The results show that there was significant gingival recession in both the test and control group (p=0.003), but no significant difference between the two groups, although, the amount of recession was seen to be greater in the test site as compared to the control site.
There was a significant bone fill obtained in the test site (p<0.001), unlike the control site, where the bone fill was negligible (percentage of defect fill: 54.69% in test site as compared to 8.91% in control site). Finally, mean changes in the clinical parameters is shown in [Table/Fig-12].
Discussion
The present study was designed to assess the clinical regenerative capacity of Ultra-Ti Titanium in the treatment of periodontal intra-bony defects. The split–mouth design was undertaken, so as to facilitate the comparison of two treatment modalities under similar healing conditions by eliminating patient–specific conditions [8,9].
This clinical study was planned for a nine month period as the previous studies have shown that the dimensional alterations of the periodontal tissues as a result of active therapy occur within six months [10,11]. The patients with bilateral two wall infra-bony osseous defects in contralateral arch were selected and each patient was subjected to recording of clinical parameters like Plaque Index, Gingival Index (GI) at baseline and nine months postoperatively. Other soft tissue parameters that were recorded were:
PPD, RAL, Gingival position/ recession (REC) at baseline and at nine months post-operatively was recorded by using acrylic stents and silver points [Table/Fig-5]. The lower border of the customized acrylic stent served as a fixed reference point. Radiographs were taken at baseline and after nine months. The surgical procedure was carried out as mentioned in the methodology.
The significant decrease seen in plaque and GI in both test and control groups could be attributed to professional oral prophylaxis at every recall visit and patient compliance [5,12].
The present study showed significant reduction in PPD for both test and control sites although the test site showed significantly increased probing pocket reduction as compared to the control site. These findings were consistent with those of Seung YS et al., [13], Cortellini et al., [5] and Tonetti et al., [14], where they had used self-supporting, space maintaining membranes like that used in this study. In the present study, the mean RAL gain was statistically significant in both the groups, although it was seen to be higher in the test site [p = 0.05]. These observations are comparable to the study reviews by Murphy et al., [15], where after carefully reviewing the studies by Cortellini et al., [5], Caffesse et al., [16] & Tonetti et al., [17], he concluded that GTR procedures resulted in greater gain in clinical attachment when compared to open flap debridement and no differences were detected among barrier types.
The studies by Cortellini et al., [5] and Tonetti et al., [14], showed greater reductions in probing depths and greater gain in attachment level i.e., 6.30 ± 1.40mm of probing depth reduction, as compared to 4.917 ± 0.996mm in this study and 5.30 ± 2.20mm gain in attachment levels, as compared to 4.375 ± 1.184mm in this study ultimately indicating that the need to create and maintain space should be a key objective of regenerative approaches based upon the principles of GTR.
Variations in results between studies may be due to many factors like methodology of probing techniques (stent vs. no stent; conventional William’s probe vs. Pressure–sensitive UNC 15 probe), the nature and extent of the defects and patients oral hygiene and compliance. Other reasons may be: Type of incisions used to raise a mucoperiosteal flap (vertical incisions vs. no vertical incisions), placement of the ePTFE–TR membranes i.e., coronal positioning of the membrane, almost 3mm coronal to the alveolar crest, unlike in this study, suturing of the flaps, so as to position them coronally, unlike in this study, where simple interrupted sutures were given, maintenance period (1 year vs. 9 month in this study).
The results show that there was significant GR in both the test and control group, but no significant difference between the two groups, although, the amount of recession was seen to be greater in the test site as compared to the control site. This result was in agreement with the studies by Javed F et al., [18], Kilic et al., [19], and Bonghade ML [20], where the test sites showed greater gingival recession as compared to the control site. There was a significant bone fill obtained in the test site, unlike the control site, where the bone fill was negligible. The percentage of defect fill was 54.68% in test site, as compared to 8.91% in control site. This observation was consistent with that of Polimeni G et al., [21] & Seung YS et al., [13] where the bone gain was 4.1 ± 1.5mm for test group and 0.5 ± 2.0mm for the control group and Wang et al., [1], who obtained approx 50% bone fill in the test site. [Table/Fig-13] shows the comparison of results obtained with other membranes. In all the studies, space provision was shown to have a significant effect on alveolar bone regeneration in periodontal sites, similar to our study where the membrane used was a self-supporting and space maintaining membrane.
Comparison of parameters obtained by ultra-ti titanium membrane, Ti-reinforced membranes, ePTFE membranes and other resorbable membranes.
| Parameters | Mean Probing Pocket Depth (mm) | Mean RAL Gain (mm) | Mean Defect Depth Reduction (mm) |
|---|
| Non-resorbable membranes | Ultra – Ti Membrane | 4.917 (test) vs. 3.833(control) | 4.375 vs. 3.417 | 3.083 Vs 0.417 (54.68% vs. 8.91%) |
| Cortellini et al., (1995) [5] | 6.3 ± 2.5 (Ti- reinforced membrane) | 5.3 ± 2.2 | 9.4 ± 3 |
| 5.5 ± 2.6 (ePTFE membrane) | 4.1 ± 1.9 | 7.9 ± 2.5 |
| Polimeni et al., 2005 [21] | -- | -- | 4.1 ± 1.5 vs. 0.5 ± 2 |
| Wang et al., [1] | -- | -- | 50% defect reduction in test site |
| Resorbable membranes | Lawrence CP et al., 2009 [25] | 3.12 ± 0.85 (Collagen membranes) | 2.37 ± 2.10 | 3.21 ± 1.15 |
| 3.21 ± 1.12 (Collagen membranes) | 2.58 ± 1.9 | 2.5 ± 1.99 |
| Lawrence CP et al., 2009 [25] | 5.64 ± 1.3 (poly-glycolic and poly-lactic acid membranes) | 3.87 ± 1.64 | 1.93 ± 1.04 |
| 3.44 (poly-glycolic and poly-lactic acid membranes) | 2.89 ± 0.9 | 2.13 ± 1.21 |
In the present study, 4 out of 12 patients, exhibited membrane exposure, but there was no negative outcome observed in the healing of the site. This was in agreement with studies done by Bonghade ML [20] in which he concluded that presence of plaque on the membranes did not compromise the initial clinical healing during the first 4-6 weeks and Novaes JR et al., suggested that, even when large portions of the membranes are exposed, contamination by periodontopathic bacteria commonly associated with destructive periodontal disease could be controlled by pre and post-operative use of antibiotics and topical chlorhexidine and thus, good regenerative results can be achieved [22]. But the results of the present study were different from the results obtained by Thomas MV [23] and Karring et al., [24], where they found a positive correlation between the membrane exposure and reduced clinical attachment level gains.
From this we see that the results obtained with Ultra-Ti Titanium membrane is comparable to the results obtained by the other resorbable and non-resorbable membranes which is in agreement with the studies by Novaes JR et al., [22] and the meta-analysis by Lawrence CP [25], where it is concluded that similar results can be achieved in GTR, from a clinical and histological standpoint, whether bioabsorbable or non-bioabsorbable barriers are applied, except for a slight decrease in CAL gain when using non-bioabsorbable membrane.
Therefore, the use of biodegradable barriers may be recommended, as surgical re-entry to remove non-resorbable barriers can be avoided.
Limitation
More number of studies using this membrane are desired since:
This was the first clinical study using this membrane and the sample size is small.
The efficacy of this membrane should be compared with other resorbable and non-resorbable membranes by conducting three split-mouth prospective research studies, without a re-entry procedure so that its ability for regeneration can be assessed and the amount of gingival recession can be reduced.
Conclusion
To conclude, it could be clinically implied from this study that by virtue of its adaptability and passivity, this membrane doesn’t require to be fixed like the other membranes and it also maintains space which is a very important pre-requisite for all membranes and thus, it could be deduced that Titanium membrane was effective in the treatment of human infra-bony defects.
*Statistical Analysis done by Student’s paired t-test
*Statistical Analysis done by Student’s paired t-test