Advancements in periodontal regenerative techniques have encouraged clinicians to seek the therapeutic goal of achieving periodontal regeneration that aims reconstruction of the tooth supporting apparatus which has been lost due to periodontitis. Over the years, numerous strategies have been implemented to surgically reconstruct intra-bony periodontal defects. In recent years, emphasis on the use of growth factors for periodontal healing is gaining great momentum. In addition, recombinant growth factors have also been made available for use in tissue engineering as a result of recent developments in molecular biology [1]. Growth factors that are considered to have a positive impact on periodontal regeneration includes Platelet Derived Growth Factor; Insulin (PDGF) Insulin like growth factor-1 (IGF-1); Fibroblast growth factor (FGF); Transforming growth factor-β; TGF-β. Several growth factors, alone [2,3] and in combination [4–7] have been tested for periodontal regeneration in various animal experiments. Various studies have shown that IGF-I in combination with PDGF stimulated periodontal regeneration in humans [8] and in experimental studies [4–7], it was found that IGF-I appears to stimulate bone formation by increasing cellular proliferation and bone matrix production. PDGF significantly promoted new attachment formation and osseous defect fill as compared to vehicle treated controls [9]. Angiogenic growth factors in particular Vascular Endothelial Growth Factor (VEGF), have also been reported to promote bone turnover [10], osteoblast migration [11] and mineralization [12]. Safety and tolerability of rh-VEGF has been tested in medical field in a clinical trial [13]. In an experimental study bone regeneration in mandibular osseous defects was observed using rh-VEGF [14]. To the best of our knowledge there is paucity of literature regarding the role of IGF-I and VEGF in solo and in combination in the management of human periodontal two wall intra-osseous defects. Hence, the purpose of the current study was to assess the clinical outcomes of rh-IGF-I and rh-VEGF with β-tricalcium phosphate β-TCP carrier and resorbable PLGA barrier membrane in human two wall intra-osseous defects.
Materials and Methods
Participant selection and study design: In this 6-month follow up, randomized, controlled clinical study, a total of 27 subjects suffering from chronic periodontitis (Males:16 Females:11) aged between 25 and 65 years with 29 intra-bony defects were selected from the Outpatient Department of Periodontology, Faculty of Dental Sciences, King George’s Medical University, Lucknow, Uttar Pradesh, India. All clinical procedures were performed in accordance with the Declaration of Helsinki and the Good Clinical Practice Guidelines. Sample size for the study was determined statistically by G power analysis software (G power v.2.0, Bonn, Germany) and power of the study was found to be 80%. Prior to initiating the study, the subjects were informed about the purpose and design of this clinical study and were required to sign an informed consent. The protocol of the study was approved by the Institutional Review Board. A thorough medical and dental history, radiographic and routine blood examination of each subject was obtained which included Hemoglobin (Hb), Bleeding Time (BT), Clotting Time (CT), Total Leukocyte Count (TLC), Differential Leukocyte Count (DLC), Hepatitis B (HbsAg) and Human Immunodeficiency Virus (HIV). The inclusion criteria included: 1) Presence of two wall intra-osseous defects which were confirmed during surgery; 2) PPD>5 mm after phase-I therapy; 3) Subjects who had not taken antibiotics within the 6 months of initial examination; 4) No periodontal surgery performed in the areas to be treated within the last 12 months. The following were the exclusion criteria: 1) Subjects having any deleterious habits such as smoking and tobacco chewing; 2) Subjects with three wall or one wall intra-osseous defects; 3) Test tooth with grade III mobility and furcation defects; 4) Untreated acute infection at surgical site; 5) Untreated carious lesion at CEJ or on root surface; 6) Pregnant or lactating women.
Pre-surgical protocol: Before the surgery, each subject was given careful instructions on proper oral hygiene measures. Instructions were repeated until the subjects maintained the satisfactory oral hygiene with plaque score ≤15% [15]. Each subject received Phase-I therapy which comprised of full mouth supra-gingival and sub-gingival scaling and root planing using hand and ultrasonic instruments. Occlusal adjustments were done if necessary to relieve traumatic occlusion. Four weeks after the scaling and root planing just prior to the surgical procedure, each subject received a re-evaluation to record the baseline data. Customized acrylic occlusal stent with a guide groove was fabricated for each study participant that was placed over the selected tooth and the adjacent teeth, so as to record the clinical measurements. This provided a fixed angulation for the measurements at each site over the entire duration of the study. Intra-osseous defects were divided into 3 test groups and 1 control group, determined randomly through the coded sealed packets [16]. Test group (n=22) comprised of group I, II and III. In group I (n=8)- defect site received β-TCP (R.T.R. Syringe, Septodont, New Castle, USA), biodegradable membrane (PLGA) (Bio Mesh®, Samyang Corporation), rh-VEGF (Reprokine™ New York, USA) and (-rh-IGF-I (Reprokine™ New York, USA) [Table/Fig-1], group II (n=7) - β-TCP, PLGA membrane and rh-VEGF [Table/Fig-2] group III (n=7) - β-TCP, PLGA membrane and rh-IGF-I [Table/Fig-3] and control group (n=7) - β-TCP and PLGA membrane [Table/Fig-4].
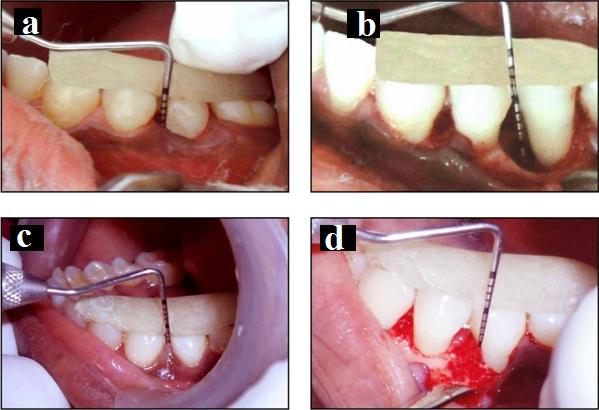
Test group I: a) Probing pocket depth at baseline; b) Intra-operative clinical image of representative intra-osseous defect; c) Probing pocket depth at 6 months; d) Surgical re-entry at 6 months.
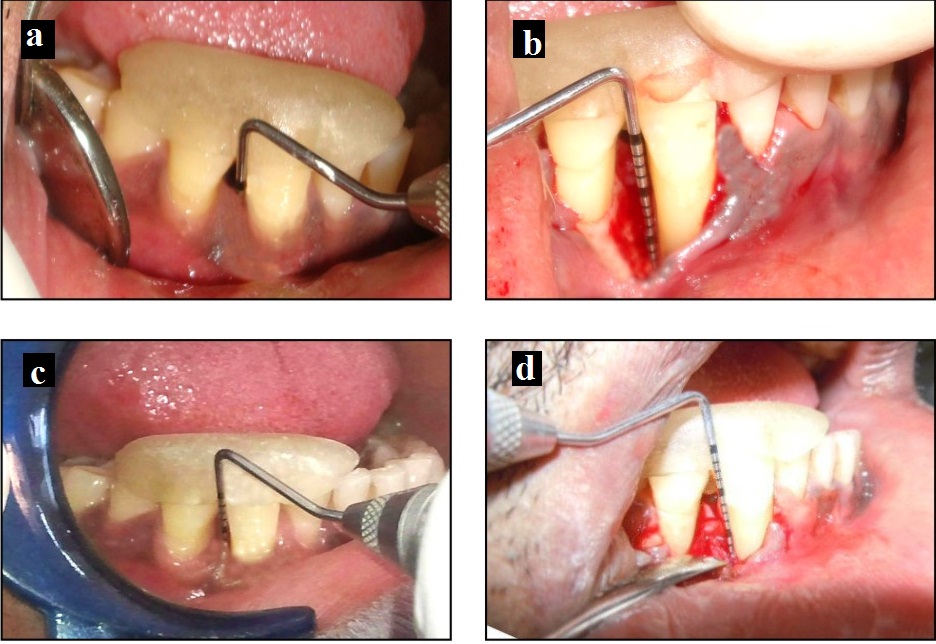
Test group II: A) Probing pocket depth at baseline; B) Intraoperatory view of the defect; C) Probing pocket depth at 6 months; D) Surgical re-entry at 6 months.
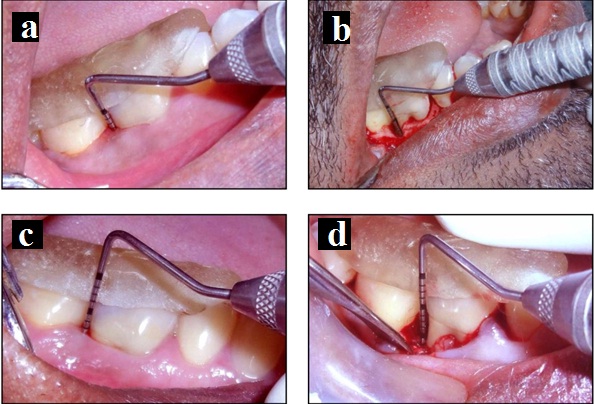
Test group III: A) Probing pocket depth at baseline; B) Intraoperative view of the defect; C) Probing pocket depth at 6 months; D) Surgical re-entry at 6 months.
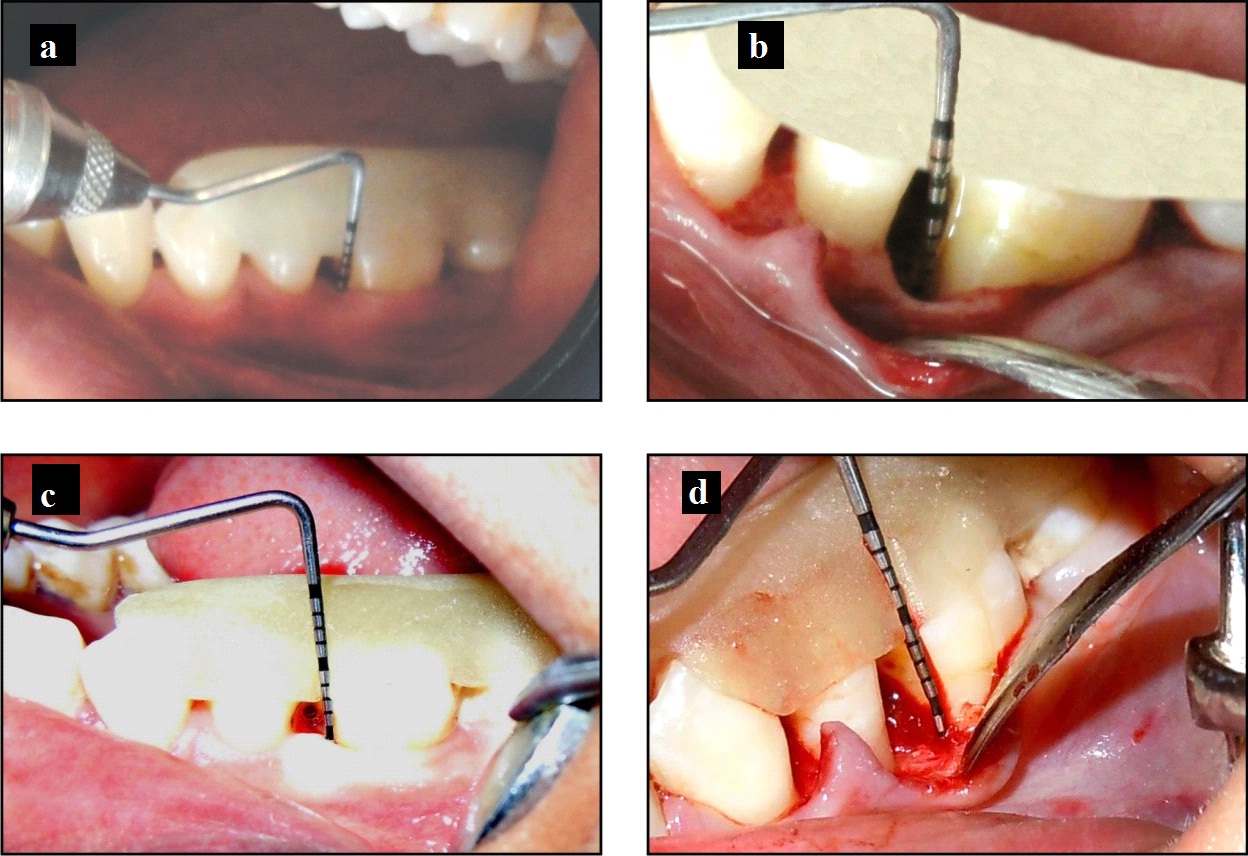
Control group: a) Probing pocket depth at baseline; b) Intraoperative view of the defect; c) Probing pocket depth at 6 months; d) Surgical re-entry at 6 months.
Clinical measures: All the soft and hard tissue measurements were recorded for the test and control group by a single investigator using a UNC-15 graduated periodontal probe (UNC-15 Periodontal Probe, Hu-Friedy, Chicago, IL) at the selected interdental site. Soft tissue measures included Probing Pocket Depth (PPD), Clinical Attachment Level (CAL) and GR. The selected inter-dental site representing the deepest point of the PPD was included in the study. Cemento-Enamel Junction (CEJ) served as the fixed reference point.
Hard tissue measurements of the intra-osseous component were calculated as: a) Distance from CEJ to the most coronal extension of the interproximal bone crest (CEJ-BC); b) Distance from bone crest to deepest interproximal point of the defect BC-BD (VDD).
Reconstitution and procurement of rh-IGF-I and rh-VEGF: The rh-IGF-I in E. Coli is a single, non-glycosylated, polypeptide chain and rh-VEGF produced in E. Coli is a double, non-glycosylated, polypeptide chain. Both rh-VEGF and rh-IGF-1 are purified by proprietary chromatographic techniques. A total of 100μg rh-IGF-I was reconstituted in 666μL sterile 18MΩ-cm water to obtain a concentration of 0.15mg/ml and 10μg rh-IGF-I was used in each subject. A total of 10μg rh-VEGF was reconstituted in 130μL sterile 18MΩ-cm water and 0.8μg rh-VEGF was used in each subject.
Surgical protocol: All the subjects were asked to rinse with 0.2% chlorhexidine gluconate for 30 second prior to surgical procedure. Area subjected to surgery was adequately anesthetized by nerve block/infiltration depending on the surgical site. After local anesthesia, sulcular incisions were made using #15 Bard parker blade up to the level of alveolar bone on the facial and palatal/lingual sides. Full thickness flap was reflected on both facial and palatal/lingual sides to allow adequate visualization of the treatment site using periosteal elevator. In all subjects, the target osseous defect was debrided of the granulation tissue using surgical curettes. The root surface was thoroughly scaled and planed with area specific Gracey’s curettes (Gracey’s Curettes, Hu-Friedy) and defect area was irrigated with sterile saline. The osseous defect was examined and only two wall intra-osseous defects were included. After debridement, direct measurements of the osseous defect were taken using a UNC-15 periodontal probe. As per the groups assigned, control group received only β-TCP and test groups received osseous graft (β-TCP) saturated with reconstituted growth factors (rh-IGF-I or rh-VEGF) for 10 minutes to permit binding of the growth factors to the osseous graft. Care was taken to protect the treatment site from saliva contamination. Each defect was filled adequately to the level of the remaining bony walls of the defect.
In all the groups, PLGA membrane was reshaped to permit precise adaptation to the interdental zone and bony defect. The PLGA membrane extended 3mm-4mm beyond the margin of the defect apically and 2mm-3mm mesio-distally and 2mm apical to the CEJ. The flaps were closed with interproximal interrupted sutures using non-resorbable 3-0 black silk sutures (Ethicon, Johnson & Johnson, Somerville NJ). Periodontal dressing (Coe-Pak, GC America, Chicago IL) was applied at the surgical site.
Post-surgical protocol: During post-operative care, Amoxicillin 500mg, three times daily for 5 days, Ibuprofen 400mg, three times daily for 5 days and Vitamin B-Complex once daily for 5 days were prescribed to all the subjects. Subjects were advised to abstain from brushing the teeth in the operated area until the suture removal, 10-14 days after surgery and to rinse with 0.2% chlorhexidine digluconate twice daily. No interdental cleaning was allowed in the treated area during the first four post-operative weeks.
Maintenance care: All subjects were on maintenance care program. They were recalled for reviewing their oral hygiene status at 1, 3 and 5 months. Requisite treatment was performed as per the subject’s treatment needs.
Re-entry: All treated sites were re-entered 6 months post-surgically. Full thickness flap was elevated and all soft and hard tissue measurements as described above were recorded. Mucoperiosteal flap was repositioned and sutured with 3-0 silk sutures. Periodontal dressing was placed, sutures and dressings were removed after 10-14 days.
Statistical Analysis
The statistical analysis was done using SPSS software (Statistical Package for Social Sciences) Version 15.0 statistical analysis software (SSPS vs. 15, IBM, Chicago, IL). The values were represented in number (%) and mean ± SD. Mann-Whitney U test was used to compare the inter-group variations. Wilcoxon signed rank test was used to test the significance of change between the pre-operative and post-operative measurements for each study group. A p-value <0.05 is considered significant.
Results
All the 27 subjects completed the study with no adverse effects and uneventful post-operative healing. Clinical measurements were recorded at baseline and at 6 months post-operatively.
Clinical Outcomes: Changes in the soft and hard tissue measures between baseline and at 6 months in both test and control groups are displayed in [Table/Fig-5].
Intra-group comparison of clinical measures (mean ±SD) (in millimeters) at baseline and 6 months (Wilcoxon signed rank test).
| Parameters | Test group I | Test group II | Test group III | Control group |
|---|
| Mean±SD | p | Mean±SD | p | Mean±SD | p | Mean±SD | p |
|---|
| Probing Pocket Depth |
| Baseline | 9.25±1.28 | 0.01* | 10.00±1.63 | 0.01* | 8.86±1.21 | 0.01* | 8.29±1.38 | 0.01* |
| 6 months | 4.63±0.74 | 6.00±1.15 | 5.00±1.00 | 5.14±1.21 |
| Mean change | 4.62±1.40 | 4.00±0.81 | 3.85±0.69 | 3.14±0.69 |
| Clinical Attachment Level |
| Baseline | 10.25±2.25 | 0.01* | 10.43±1.99 | 0.01* | 9.57±2.37 | 0.01* | 8.71±1.38 | 0.01* |
| 6 months | 5.88±1.64 | 6.43±1.62 | 5.71±2.14 | 5.71±1.25 |
| Mean change | 4.37±1.68 | 4.00±0.81 | 3.86±0.69 | 3.00±0.57 |
| Gingival Recession |
| Baseline | 1.00±1.51 | 0.31 | 0.43±1.13 | 1.0 | 0.71±1.50 | 1.0 | 0.43±0.79 | 0.31 |
| 6 months | 1.25±1.49 | 0.43±1.13 | 0.71±1.50 | 0.57±0.98 |
| Mean change | 0.25±0.71 | 0 | 0 | 0.14±0.38 |
| Vertical Defect Depth |
| Baseline | 6.00±0.93 | 0.01* | 6.00±0.82 | 0.01* | 5.43±0.79 | 0.01* | 5.57±0.98 | 0.01* |
| 6 months | 0.25±0.71 | 2.71±0.49 | 2.57±0.79 | 3.29±1.25 |
| Mean change | 5.75±0.89 | 3.29±0.76 | 2.86±0.69 | 2.29±0.95 |
| CEJ to BC |
| Baseline | 5.13±1.36 | 0 | 4.14±0.90 | 0 | 4.43±1.72 | 0 | 2.86±1.07 | 0 |
| 6 months | 5.13±1.36 | 4.14±0.90 | 4.43±1.72 | 2.86±1.07 |
| Mean change | 0 | 0 | 0 | 0 |
* Denotes significant difference.
1. Probing Pocket Depth (PPD): After 6 months, the maximum mean reduction of PPD was seen in Test group I (4.62±1.40mm) and minimum reduction (3.14±0.69mm) in the control group. In Test group II and III mean values of PPD was 4.00±0.81mm and 3.85±0.69 mm respectively. Intragroup comparison revealed that mean reduction in PPD was significant (p<0.05) in all the groups [Table/Fig-5]. After 6 months, on intergroup comparison, only significant changes (p<0.05) in pocket depth reduction was seen test group I vs. II [Table/Fig-6].
Between group comparison of the clinical measures after 6 months. (Mann-Whitney U test).
| Comparison | Statistic | PPD | CAL | GR | VDD(BC to BD) | CEJ to BC |
|---|
| Test group I vs Control | z | 0.678 | 0.000 | 0.935 | 3.427 | 2.847 |
| p | 0.536 | 1.000 | 0.463 | 0.001* | 0.004 |
| Test group I vs II | z | 2.270 | 0.722 | 1.246 | 3.375 | 1.403 |
| p | 0.029* | 0.536 | 0.336 | 0.001* | 0.189 |
| Test group I vs III | z | 0.680 | 0.414 | 0.853 | 3.240 | 1.390 |
| p | 0.536 | 0.694 | 0.463 | 0.001* | 0.189 |
| Test group II vs III | z | 1.662 | 1.116 | 0.622 | 0.643 | 0.134 |
| p | 0.128 | 0.318 | 0.710 | 0.620 | 0.902 |
| Test group II vs Control | z | 1.324 | 0.930 | 0.445 | 0.967 | 2.061 |
| p | 0.209 | 0.383 | 0.805 | 0.456 | 0.053 |
| Test group III vs Control | z | 0.205 | 0.546 | 0.001 | 1.330 | 1.887 |
| p | 0.902 | 0.620 | 1.000 | 0.259 | 0.073 |
*Denotes significant difference
2. Clinical Attachment Level (CAL): Statistically significant (p<0.05) mean changes in CAL gain were found in Test group I (4.37±1.68 mm), 4.00±0.81mm in test group II, 3.86±0.69 mm in test group III and 3.00±0.57amm in control group at six months. No statistically significant changes were seen on intergroup comparison in clinical attachment gain after six months.
3. Gingival Recession (GR): Non-significant (p>0.05) gingival recession of 0.14±0.38mm and 0.25±0.71mm was observed in the control and Test group I at 6 months whereas in both Test groups II and III, the mean values remained constant from baseline to 6 months. On intergroup comparison, no significant changes were found after 6 months.
4. Vertical Defect Depth (VDD): Statistically significant defect fill (p<0.05) was present in both test and control groups from baseline to six months. Test group I exhibited maximum defect fill of 5.75±0.89mm (95.8%) followed by 3.29±0.76mm (54.8%) in Test group II and 2.86±0.69mm in Test group III (52.7%). 2.29±0.95mm (41.1%) defect fill was present in control group [Table/Fig-5]. Between group comparison (Whilcoxon sign rank test) showed highly significant values (p=0.001) when Test group I was compared with Test group II, III and control group whereas the values in Test group II vs. III, and in control group vs. Test group II and III were found to be non-significant (p>0.05) [Table/Fig-6].
5. Distance from CEJ to BC remained unchanged from baseline to 6 months in all the tests and control group.
Discussion
Results of this randomized controlled clinical study showed that combined use of rh-VEGF and rh-IGF-I in conjunction with PLGA membrane and β-TCP osseous graft resulted in enhanced clinical outcomes in terms of pocket reduction, CAL gain and bone fill as compared to rh-VEGF and rh-IGF-I used alone.
In the present study results of soft tissue parameters in the control group are only compared with the previous studies but with a difference i.e., in control group β-TCP was used along with PLGA membrane whereas in previous studies either β-TCP [17,18] or barrier membrane [19,20] was used.
Results of growth factors in our study are not directly comparable with other studies because firstly earlier studies were conducted either in-vitro [21–23] or in animals [24–27] and secondly in humans hard tissue parameters were considered [8]. Most of the studies on the growth factors comprised of combination of PDGF/IGF-I in periodontal regeneration as compared to rh-VEGF. Therefore, in the present study, we tried to corroborate our results with in-vitro and experimental studies.
In the present study 0.15mg/ml concentration of rh-IGF-I resulted in more than 50% osseous fill. Though, approximately similar concentration was used in the previous study but they observed the maximum mitogenic and chemotactic effect of IGF-I in-vitro [21]. IGF-I in different concentrations 50-200ng/ml [22] and 25ng/ml [23] were also used in various in-vitro studies for the assessment of mitogenic response. Periodontal regeneration was also observed in monkeys using IGF-I at a dose of 5μg [4] and 10μg [9], and in beagle dogs at a concentration of 1μg [5] and 3μg [6]. The 0.8μg rh-VEGF used in our study concurs with an experimental study which revealed more angiogenesis and bone regeneration with similar dose [24]. In contrast, other authors also observed angiogenesis using 3μg [25] and 5μg/ml [26] of rh-VEGF in their experimental studies. Concentration of 0.05μg/μl murine rVEGF revealed osteogenesis in an animal study [27].
Although both bioresorbable and non-resorbable membranes resulted in comparable results in intra-osseous defects but, the use of bioresorbable membranes seems to be gaining popularity in recent years. A biodegradable barrier membrane that is formed by copolymerization of polylactic acid and polyglycolic acid (PLGA) was selected for our study as PLGA membrane with interconnective porous structure promoted good nutrient flow, blood vessel formation, cell occlusiveness and biodegradability [28]. As per the previous study of Yang P et al., β-TCP was used as scaffold for optimal bone formation and to prevent wash out of growth factors which maintain the concentration at the defect site for a sufficient period of time to allow bone-forming cells to migrate to the site of repair, proliferate and differentiate in response to growth factors [25].
Mean PPD reduction was found to be significant (p<0.05) at the end of the study period in the control group (3.14mm). This is in partial agreement with the previous studies using β-TCP [29,30] and PLGA membrane only. CAL gain in the control group (3.00mm) was comparable with the previous studies. CAL gain was slightly less than 3.35mm and more than 1.75mm observed in the previous studies of using PLGA membrane only. A 0.14mm of mean GR was observed in control group at 6 months.
Significant (p<0.05) mean reduction in VDD or osseous fill was observed in all the groups. In rh-IGF-I treated sites VDD reduction was 2.86mm (52.7%) which was 0.57mm more than that of control group (2.29mm) (41.1%) thus, suggesting the positive interaction between rh-IGF-I and osteoblasts. A 7-day time course of IGF-I infusion increased both cortical and trabecular bone formation, while stimulating osteoblast proliferation and decreasing osteoclast number in rats [31].
A histomorphometric study illustrated that the treatment with IGF-I at a dose of 10μg resulted in 13.1±5.4% and 24.7±11.5% Osseous Defect Fill (ODF) at 1 and 3 months respectively [9]. An in-vitro study Matsuda N et al., [21] revealed that IGF-I promoted bone matrix apposition (i.e., collagenous protein synthesis), had modest effects on osteoblast, PDL mitogenesis, chemotactic for PDL fibroblasts and osteoblasts and binding of IGF-I to a membrane bound-receptor stimulated the receptor tyrosine kinase, thus, generating a signal that resulted in a pleiotropic cellular response [32]. Recently, it was suggested by Yu Y et al., [33] that IGF-I was a potent agent for stem cell based periodontal tissue regeneration. In an animal study, Ortolani E et al., [34] demonstrated that combination of PDGF/IGF-1 resulted in more osseointegration in implants when compared with PRP-treated or controls.
The rh-VEGF treated sites showed VDD reduction of 3.29mm (54.8%), 0.43mm higher than rh-IGF-I treated sites (2.86 mm) (52.7%). This may be due to enhanced angiogenesis in the defect area leading to improved osseous fill. Role of VEGF in promoting bone formation in the osseous defect could also be due to its binding to specific tyrosine kinase receptors [35]. Street J et al., [10] demonstrated enhanced blood vessel formation, ossification and new bone maturation when exogenous VEGF was applied to the rabbit radius segmental gap defect. The results of the Murphy WL et al., [12] supported the concepts of both angiogenesis and subsequent bone regeneration. They also suggested the differentiation of infiltrating osteoblasts and osteoblast precursor cells during new bone development because of angiogenesis. Yang P et al., [25] in their study documented that a critical sized defect in the rabbit radii treated with 3μg rh-VEGF incorporated in porous β-TCP scaffolds by Fibrin Sealant (FS) could be completely bridged with cortical bone in 12 weeks whereas our study exemplified that 0.8μg of rh-VEGF+ β-TCP + membrane resulted in 54.8 % (3.29mm) as compared to 41.1% (2.29mm) in β-TCP+membrane group in 6 months suggesting that β-TCP scaffold is accelerated in the presence of rh-VEGF. Weibing Z et al., [36] investigated the expression of VEGF during midpalatal suture expansion and observed increased vascularization and subsequent new bone formation during rapid maxillary expansion.
In an animal study, it was demonstrated that β-TCP with Bone Marrow Derived Mesenchymal Stem Cells (BM-MSCs) and biodegradable PLGA microspheres containing Bone Morphogenetic Protein-2 (BMP-2) and VEGF treated mandibular defects had higher tissue mineral densities than β-TCP only and β-TCP+BM-MSC’s only [37].
Although rh-IGF-I and rh-VEGF in solo promoted more than 50% osseous fill but nonetheless the combination of growth factors (rh-IGF-I + rh-VEGF) resulted in 95.8% osseous fill after 6 months re-entry. The data thus demonstrated that short term exposure of growth factors in osseous defect could stimulate a cascade of wound healing events leading to bone formation in two wall intra-osseous defects. It can be speculated that the application of rh-IGF-I and rh-VEGF in a dose of 10μg and 0.8μg respectively may have increased the number of osteoblasts. Pfeilshifter J et al., used three different growth factors PDGF, IGF-I and TGF-β and opined of a positive interaction between them [38]. Likewise, the present study also supports the hypothesis of positive interaction between rh-IGF-I and rh-VEGF by promoting significantly more bone in the osseous defect than the growth factors individually. A clinical study of Howell TH et al., [8] demonstrated that PDGF/IGF-I subjects resulted in 42.3±9% bone fill at a dose of 150μg/ml at 9 months re-entry whereas in the current study rh-IGF-1 in combination with rh-VEGF resulted in 95.8% bone fill after 6 months. A preliminary report in Beagle dogs [7] compared the effects of IGF-I alone to PDGF-BB/IGF-I and surgery alone and found that IGF-I treated sites demonstrated less periodontal regeneration than PDGF-BB/IGF-I treated sites but revealed slight increase in bone formation and osteoblast number than surgery alone. In our study rh-IGF-I treated sites also exhibited less osseous fill than rh-IGF-I+rhVEGF treated sites.
In the combination group of growth factors i.e., Test group I, it was noteworthy that the mean defect of 6.00mm at baseline was reduced to 0.25mm after 6 months [Table/Fig-1]. It was further interesting to note that out of 8 subjects in this group 100% bone fill was observed in 7 subjects and in one subject 2mm of osseous defect remained unfilled. This can be due to the presence of plaque at the treated site as the subject did not follow the oral hygiene regimen strictly as compared to other 7 subjects. The synergistic effect of rh-VEGF and rh-IGF-I can be explained on the basis of the direct effect of VEGF on osteoblast to promote osteoblast migration, proliferation and differentiaition in an autocrine manner and indirect effect via endothelial cells as documented by Hsiong SX et al., [39] and secondly VEGF is also a mediator of many osteoinductive factors, including insulin IGF-I which upregulates VEGF expression in osteoblasts [40,41]. No crestal resorption was observed in any of the groups. This is in accordance with the previous study of Snyder AJ et al., [18].
Clinical implications of the present study and future perspectives: The dawn of regenerative therapy has apparently made periodontal regeneration a certainty and thus, it is the treatment of choice for intra-osseous defects in present-day clinical practice. The rh-VEGF and rh-IGF-1 when combined with bone graft material and suitable barrier membrane seemed to be beneficial for the treatment of intra-osseous defects. To the best of our knowledge, this study is the first to report the use of the rh-VEGF to induce periodontal regeneration. Further comparative clinical studies need to be performed on rh-IGF-1 and rh-VEGF to elucidate the requirements for predictable regeneration, with large sample size.
Limitation
Limitations of the present study included assessment of periodontal regeneration using only clinical parameters. However, for the assessment of true periodontal regeneration histological evaluation is mandatory which was not performed in the present study owing to ethical concerns. Radiographs were also not taken in the study because radiographs provide only two dimentional images of three dimensional structures. Evaluation of radiographs tends to underestimate the extent of alveolar bone loss as compared to the gold standard of intrasurgical measurements [42–44].
Conclusion
Within the limitations of the present study, it can be concluded that rh-IGF-I+rh-VEGF treated sites resulted in greater improvement in PPD reduction, CAL gain as well as in osseous fill after 6 months when compared with rh-VEGF, rh-IGF-I and control sites. However, future expanded studies utilizing the information gained from this study evaluating the efficacy of rh-IGF-I/rh-VEGF in two wall intra-osseous defects are warranted to further explore the role of these growth factors in large number of subjects.
* Denotes significant difference.
*Denotes significant difference