Introduction
The Medical Council of India (MCI) has guidelines for physicians to prescribe drugs with generic names. But physicians and patients might have prejudices against generic drug substitution or concerns regarding quality and generics effectiveness. If the drugs are ineffective, they can result in adverse clinical outcomes such as treatment failure. According to WHO, β-lactams are the most common substandard drugs to be produced amongst antibiotics.
Aim
To evaluate and compare invitro efficacy of generic and branded preparations of Amoxicillin with Potassium Clavulanate.
Materials and Methods
One generic (C) and 5 branded formulations (A,B,D,E,F) of amoxicillin with potassium clavulanate were taken for microbiological assay. Coding was done. Sterile disks were instilled with 10μl of preparations and disk diffusion was screened by Kirby Bauer Method using Mueller Hinton Agar. Bacterial Strains used were Staphylococcus aureus (S.aureus) and Escherichia Coli. Zone of inhibition was measured. Statistical Analysis was done using repeated measures one-way ANOVA followed by Tukey’s test.
Results
Disk Diffusion test showed that branded Drug F has statistically significant less zone of inhibition (p < 0.001) for S. Aureus and (p < 0.05) for E.coli in comparison with generic drug C. Zone of inhibition of branded Drug A, B & E was comparable with the generic drug C.
Conclusion
The results of this study indicate that the generic drug tested was equally effective compared to the tested branded drugs except branded Drug D and F. This suggests that efficacy of generic drug is equivalent to branded drugs and maybe used interchangeably with branded drugs.
Antibiotic, Disk diffusion, Microbiological assay
Introduction
As the debate of replacing branded drugs with generic drugs heats up, it also brings the issue of substandard drugs in highlight. Physicians and patients have prejudices against substitution of generic drug and there are concerns regarding quality and effectiveness of these drugs [1]. In 2013, when the Medical Council of India (MCI) appealed to doctors to prescribe generics whenever possible, the Indian Medical Association responded saying that it requires guarantees on the quality of generic forms of drugs [2]. Therefore, it is necessary to establish that generic drugs are equivalent to branded products.
India is among the largest manufacturers of generic drugs for export to US and Europe, but generic drugs are not prescribed widely in India [3]. There is reluctance from doctors due to a negative perception in their minds to prescribe the drugs and doubt in the mind of the patients receiving such drugs [4]. This doubt emerges due to questions about safety and quality of the generic drugs. Also, the low availability of generic drugs in the retail market is a key factor [5].
Many countries, such as those of USA, Europe, Canada and South Africa require generic drug manufacturers to prove their formulation exhibits bioequivalence to the innovator product [6].
Substandard drugs are generally regarded as medicines which have not passed the standards and quality testing protocol set for them according to the International Pharmacopoieas and WHO [7,8]. Though these drugs do not have a standard and uniform definition, the WHO has defined them as “drugs that are deliberately and fraudulently mislabeled with respect to identity and/or source” [9].
In a recent literature review, out of 163 counterfeit antibiotics identified until 2009, 50% were beta-lactams. β-lactams are the most common substandard drugs to be produced amongst antibiotics [10,11].
Recently it was seen in Chhattisgarh where 13 patients died after a sterilization camp, after receiving substandard drugs including Ciprofloxacin [12]. This shows the fatal outcome of the use of substandard drugs.
Amoxicillin is a semisynthetic antibiotic with an extended spectrum of bactericidal activity against many gram-positive and gram-negative microorganisms [13]. A structurally related β-lactam, potassium clavulanate inactivates a wide range of β-lactamase enzymes commonly found in microorganisms resistant to penicillins. The formulation of amoxicillin and potassium clavulanate protects amoxicillin from degradation by β-lactamase enzymes. This results into an extended antibiotic spectrum of amoxicillin. This particular combination was chosen in this study, as it is a commonly used prescription drug for various ailments such as infections of the ears, lungs, sinus, skin, and urinary tract, due to its wide spectrum, high efficacy, low cost and less toxicity.
Antimicrobial drugs of low quality cause increased burden of disease, which lead due to excess mortality and morbidity because they result in various untoward clinical outcomes such as lack of effect, treatment failure, bacterial resistance and side effects [10].
The present study was undertaken with the aim to compare the efficacy of generic and branded preparations of amoxicillin with potassium clavulanate available in the market.
Materials and Methods
The study was conducted in October 2015, 1 generic and 5 brand preparations of Amoxicillin with Potassium Clavulanate of various multinational and indigenous manufacturers were procured from retail pharmacies of Pune, Maharashtra. All samples were kept in manufacturer’s original packing and were stored as per manufacturer’s directions until testing. Testing of samples was done within a month of procuring the samples. All the drugs had different manufacturers and distributors.
Bacterial Strains
Two bacterial strains were used in the study to determine microbial efficacy. Standard strains of Escherichia coli (ATCC 25922) and Staphylococcus aureus (ATCC 25923) were used in the study according to Clinical and Laboratory Standards Institute (CLSI) guidelines [14,15].
Microbiological Assay
Standard strains of Escherichia coli (ATCC 25922) and Staphylococcus aureus (ATCC 25923) were tested for Amoxicillin with Potassium clavulanate sensitivity by disk diffusion. Sensitivity was screened by Kirby Bauer disk diffusion method using Mueller Hinton Agar. The media was prepared according to the standard CLSI guideline that is, 20 ml of the medium was poured into a 9 cm diameter petri dish. Three to four colonies of pure culture was inoculated into peptone water broth and incubated at 37°C for 3 hour and adjusted to 0.5 McFarland standards.
This broth culture was then spread evenly on Mueller Hinton Agar plates to get a lawn of growth. 10μl of the drug was instilled onto 6 mm disks of Whatman No 1 filter paper and tested. Zone of inhibition was measured with a round diameter scale in mm after 24 h of incubation.
Statistical Analysis was done using repeated measures one-way ANOVA followed by Tukey’s test. All data in figures is shown as Mean±SD.
Results
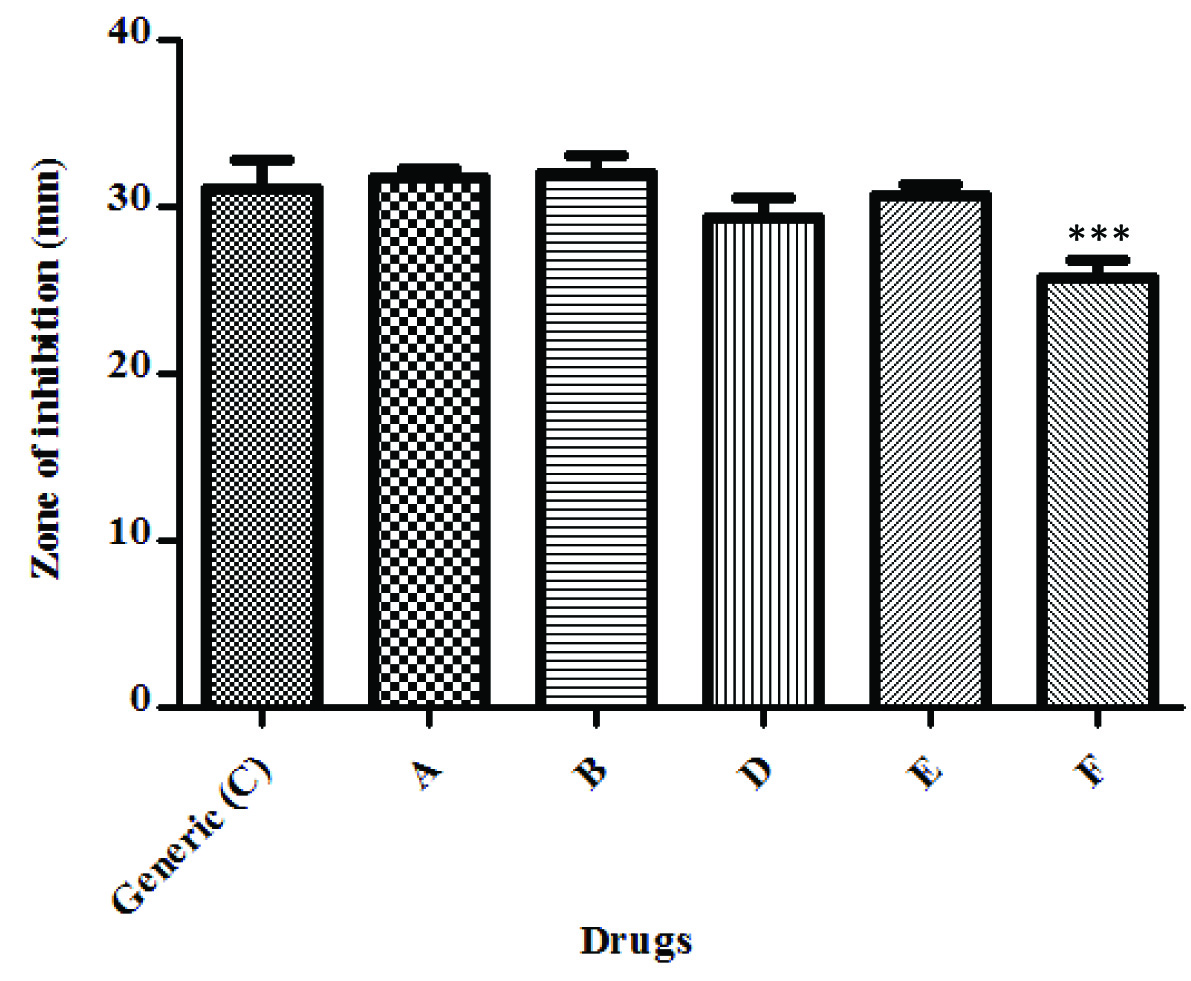
Disk diffusion test using E.Coli showed that all branded drugs, except branded drug F were comparable to generic drug C as shown in [Table/Fig-1]. Branded Drug F showed a statistically significant less zone of inhibition (***p<0.001) compared to Generic drug C and branded drug A and B. Generic drug was comparable to all other branded drugs.
Disk diffusion test - E. coli. One way repeated measures ANOVA; *** p < 0.001 when compared with Generic C, A and B.
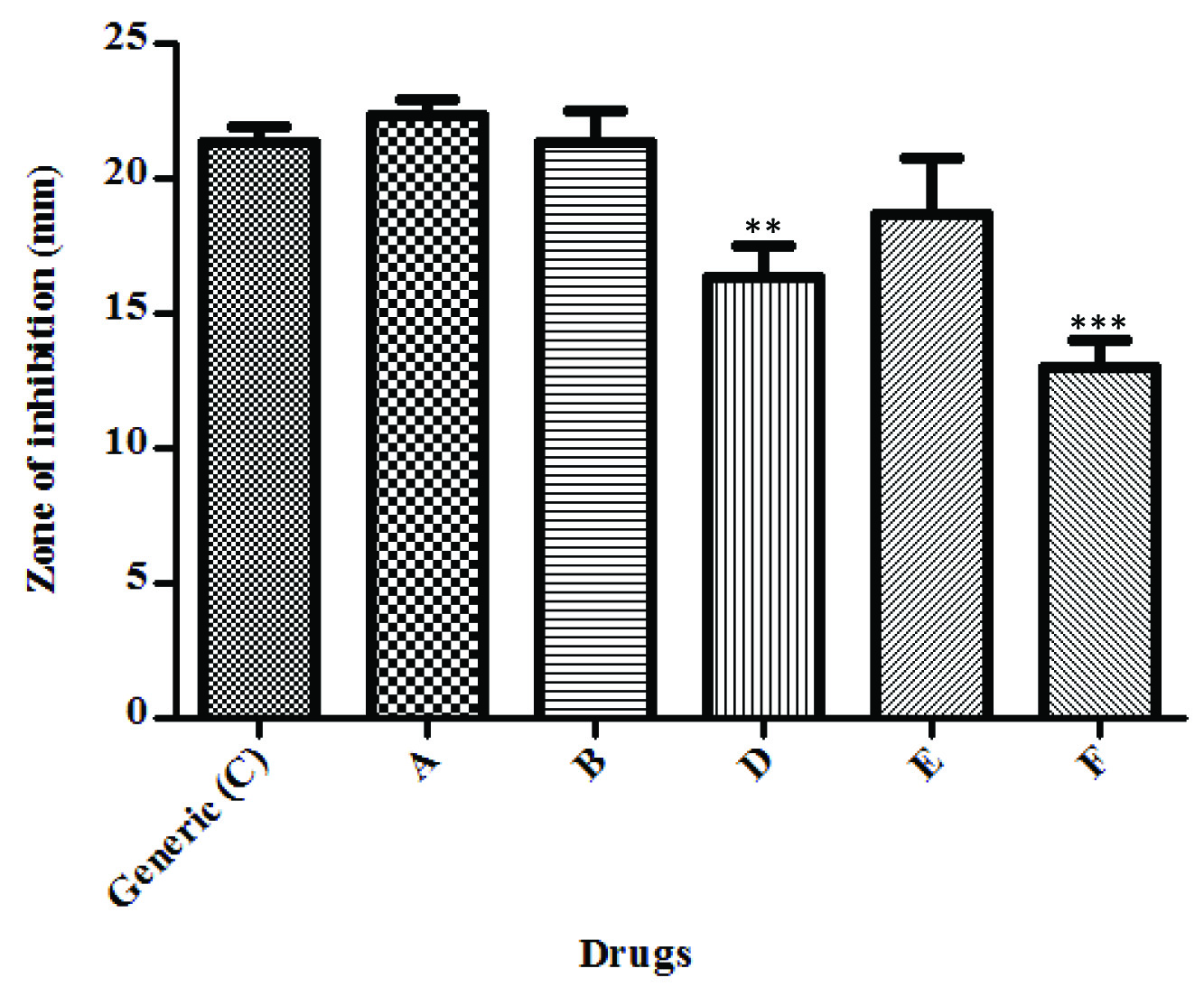
Similar results were seen in the Disk Diffusion test using Staphylococcus aureus where branded drug F showed a statistically significant less zone of inhibition (***p<0.001) compared to generic drug C and branded drug A and B, seen in [Table/Fig-2]. Also, branded drug D showed a significant difference (**p<0.01) in the zone of inhibition when compared to generic C, branded drugs A and B. The generic drug was comparable to all the other branded drugs (A,B,E).
Disk diffusion test - S. aureus. One way repeated measures ANOVA; ***p < 0.001 when compared with generic C, A and B. **p <0.01 when compared with Generic C, A and B.
Discussion
Central Drugs Standard Control Organization (CDSCO) has made stringent rules and regulations in bringing out generic medicines in public use in India, requiring extensive invitro and invivo testing before marketing and thus there is no reason to believe that generics are substandard than branded [16,17].
For India generics are very important considering our country’s socioeconomic status. The lower cost is the key advantage of generic drugs. Generic drugs are cost saving as there are no costly clinical trials and marketing involved. Getting a new drug into market costs approx $2,558 million, according to a study by the Tufts Center for the Study of Drug Development [18].
Despite the advantages of generic drugs, the reluctance among doctors to prescribe such drugs as there is a residing doubt in the patient’s mind about the efficacy of the drug [19].
The present study used disk diffusion method to test the efficacy of the different preparations on 2 different strains of organisms. The disk diffusion test was used because it is simple, practical, cost effective and well standardized. Disk diffusion test results are qualitative, that is the degree of susceptibility is obtained [20].
In our study, when we compared the generic and branded formulation of Amoxicillin with Potassium Clavulanate on two different strains it was observed that there was a statistically significant difference between efficacy of branded drug F compared to generic drug. In comparison with the branded drug F, generic drug C appeared to be more effective in increasing the zone of inhibition. Here generic drug showed more efficacy against both E. coli and S. aureus.
The generic drug C also showed a greater zone of inhibition against S. aureus when compared to branded drug D. The generic drug had comparable efficacy with Brands A, B and E. Kevin PP et al., and Odulaja J also found generic and branded amoxicillin - potassium clavulanate combinations comparable [21,22]. This correlates with the above stated studies.
Silva et al., and Jones et al., found invitro efficacy of other antimicrobial generic drugs comparable to brand drugs [23,24]. Kassaye L also compared invitro dissolution profiles for different brands of amoxicillin capsules and found most generic brands of amoxicillin capsules (62.5%) were not interchangeable with the innovator brand (Amoxil) [25].
Various other studies such as Rodriguez et al., Moet et al., observed decrease in the invitro potency of other beta lactam generic drugs compared to brand [26,27]. Some of the above stated results suggest that in spite of rigorous and strict guidelines, substandard drugs are present in the market.
Substandard drugs are a threat to public health, as they can spread resistance and prolong duration of infection and economic burden. The first State of the World’s Antibiotics report 2015, revealed by Washington-based Centre for Disease Dynamics, Economics and Policy (CDDEP) reports a steep increase in MRSA recorded by a large private laboratory network, from 29% of Staphylococcus aureus isolates in 2009 to 47% in 2014 [28]. Thus, it is imperative that India steps up its regulatory infrastructure and ensures quality drugs [29].
Limitation
All generic and branded preparations available in the market were not included in the study. Though invitro studies are cost effective and can directly assess the drug performance, further invivo bioequivalence studies would corroborate the above results.
Conclusion
Though, this study needs to be further substantiated with invivo bioequivalence testing and also therapeutic equivalence reporting, the outcome of the present invitro study highlights that the generic drug tested was equally effective as brand-name drugs. Also, with branded drug showing less effectiveness compared to other drugs shows that all drugs need to undergo extensive testing before being distributed.
[1]. Paveliu MS, Bengea S, Paveliu FS, Generic substitution issues: brand-generic substitution, generic-generic substitution, and generic substitution of Narrow Therapeutic Index (NTI)/critical dose drugsMaedica 2011 6(1):52 [Google Scholar]
[2]. Mukherjee R. Prescribe generic drugs: MCI to doctors 2013. Available at articles. timesofindia. indiatimes.com [Google Scholar]
[3]. The Associated Chambers of Commerce & Industry of India. Indian pharmaceutical industry likely to touch USD 55 bln by 2020: study. 2015. Available from: http://www.assocham.org/newsdetail.php?id=5406 [Google Scholar]
[4]. Shrank WH, Liberman JN, Fischer MA, Girdish C, Brennan TA, Choudhry NK, Physician perceptions about generic drugsAnnals of Pharmacotherapy 2011 45(1):31-38. [Google Scholar]
[5]. Bera A, Mukherjee A, The importance of generic drugs in IndiaInt J Pharm Chem Biol Sci (IJPCBS) 2012 2(4):575-87. [Google Scholar]
[6]. Shah K, Kumar S, Maheshwari KK, Bridging the gap of Indian Regulations and major global regulations for bioequivalence studies with emphasis on adaptive sequential design and two stage bioequivalence studiesJournal of Pharmaceutical and Scientific Innovation 2013 2(2):14-19. [Google Scholar]
[7]. World Health Organization. 1999. Quality assurance of pharmaceuticals, vol 2 [Google Scholar]
[8]. World Health Organization, Geneva, Switzerland. World Health Organization. 2014. What encourages counterfeiting of drugs? Available from: http://www.who.int/medicines/services/counterfeit/faqs/15/en/ [Google Scholar]
[9]. World Health Organization. 1999. Counterfeit drugs: guidelines for the development of measures to combat counterfeit drugs. WHO, Geneva, Switzerland [Google Scholar]
[10]. Kelesidis T, Falagas ME, Substandard/counterfeit antimicrobial drugsClinical Microbiology Reviews 2015 28(2):443-64. [Google Scholar]
[11]. Delepierre A, Gayot A, Carpentier A, Update on counterfeit antibiotics worldwide; public health risksMedecine ET Maladies Infectie Uses 2012 42(6):247-55. [Google Scholar]
[12]. Bagcchi S, Medical negligence and substandard drugs caused deaths in Indian sterilisation programme, report findsBMJ 2015 351:h4813 [Google Scholar]
[13]. Knollmann BC, Goodman & Gilman’s the pharmacological basis of therapeutics 2011 Sep 20 New YorkMcGraw-Hill Medical [Google Scholar]
[14]. Clinical and Laboratory Standards Institute. 2014. Performance standards for antimicrobial susceptibility testing; 24th informational supplement. CLSI document M100-S24. Clinical and Laboratory Standards Institute, Wayne, PA [Google Scholar]
[15]. Jorgensen JH, Turnidge JD, Susceptibility Test Methods: Dilution and Disk Diffusion. In Jorgensen JH, Pfaller MA, Carroll KC, Funke G, Landry ML, Richter SS, Warnock DW, editorsManual of Clinical Microbiology 2015 11th edASM Press:1253-1273. [Google Scholar]
[16]. Kirtikumar N, Maheshwari D, Documentation requirements for generic drug application to be marketed in india- a reviewJournal of Pharmaceutical Science and Bioscientific Research 2014 4(4):237-42. [Google Scholar]
[17]. Document No. - CT/71108 Version – 1.1. Guidance for Industry Submission of Clinical Trial Application for Evaluating Safety and Efficacy. Available from: http://www.cdsco.nic.in/writereaddata/cdsco-guidanceforindustry.pdf [Google Scholar]
[18]. Tufts Centre for the Study of Drug Development. Cost to Develop and Win Marketing Approval for a New Drug Is $2.6 Billion [Internet]. 2014. Available from: http://csdd.tufts.edu/news/complete_story/pr_tufts_csdd_2014_cost_study [Google Scholar]
[19]. Mudur G, Doctors in India defy guidelines on generic drugsBMJ 2013 347:f4244 [Google Scholar]
[20]. Reller LB, Weinstein M, Jorgensen JH, Ferraro MJ, Antimicrobial susceptibility testing: a review of general principles and contemporary practicesClinical Infectious Diseases 2009 49(11):1749-55. [Google Scholar]
[21]. Kevin PP, Panchmal GS, Shenoy R, Tellis R, Jacob JM, Rekha PD, Comparison of medicine quality of the generic formulation of amoxicillin provided by the Government of Karnataka with marketed brands-A public health perspectiveInternational Journal of Pharmaceutical Sciences Review and Research 2013 23(1):3-42. [Google Scholar]
[22]. Olanrewaju OJ, Paul AC, Olusola AM, Quality assessment of amoxicillin-clavulanate potassium tablets in Lagos, NigeriaJournal of Chemical and Paharmaceutical Research 2012 4:5032-38. [Google Scholar]
[23]. Silva E, Diaz J, Arias M, Hernandez A, de la Torre A, Comparative invitro study of the antimicrobial activities of different commercial antibiotic products for intravenous administrationBMC Clinical Pharmacology 2010 10(1):3 [Google Scholar]
[24]. Jones R, Fritsche T, Moet G, Invitro potency evaluations of various piperacillin/tazobactam generic products compared to the contemporary branded (Zosyn®, Wyeth) formulationDiagnostic Microbiology and Infectious Disease 2008 61(3):366-68. [Google Scholar]
[25]. Kassaye L, Genete G, Evaluation and comparison of invitro dissolution profiles for different brands of amoxicillin capsulesAfrican Health Sciences 2013 13(2):369-75. [Google Scholar]
[26]. Rodriguez C, Agudelo M, Zuluaga A, Vesga O, Invitro and invivo comparison of the anti-staphylococcal efficacy of generic products and the innovator of oxacillinBMC Infect Dis 2010 10(1):153 [Google Scholar]
[27]. Moet G, Watters A, Sader H, Jones R, Expanded studies of piperacillin/tazobactam formulations: variations among branded product lots and assessment of 46 generic lotsDiagnostic Microbiology and Infectious Disease 2009 65(3):319-322. [Google Scholar]
[28]. Gelbrand H, Miller-Petrie M, Pant S, Gandra S, Levinson J, Barter D, The State of the World’s Antibiotics 2015Wound Healing Southern Africa 2015 8(2):30-34. [Google Scholar]
[29]. Sinha K, “Antibiotic addict’ India losing fight against lethal bacteriaThe Times of India 2015 [Google Scholar]