Indazoles are emerging as pharmaceuticals with specific use in certain diseases. Investigations carried out in recent decades have identified the potential usefulness of these compounds in several biological conditions such as inhibition of apoptosis [1], treatment of rheumatoid arthritis [2], anti-proliferative activity [3], treatment of hypertension [4], anti-psychotic activity [5], hypotensive activity [6], treatment of obesity [7], tumour cell cytotoxic assays [8], anti - hyperlipidemic activity [9], trichomonacidal activity [10], Analgesic and antipyretic activity [11] and anti-inflammatory activity [12]. In our previous study, indazole, 5 -aminoindazole and 6–nitroindazole were very effective in attenuating nociceptive response especially in the inflammatory models of pain like acetic acid induced abdominal constrictions and intraplantar formalin - induced nociception [13]. Therefore, based on aforementioned literature it was considered interesting to explore the anti - inflammatory potential of indazole and derivatives of indazole viz. 5 – aminoindazole, 6 – nitroindazole in experimental animals.
Materials and Methods
Animals
Adult male Wistar rats weighing 100 – 150g were used in the present study and these were obtained from the Kings Institute of Preventive Medicine, Gundy. The animals had free access to food and water and maintained at 24 ± 1° C temperature with 12h day/12h night cycle. All the experiments were carried out between 09.00 and 13.00 h to avoid circadian variation. The experiments were carried out in Sree Balaji Medical College and Hospital, Chennai from November 2014 to April 2015. The experimental protocol was approved by the institutional animal ethical committee on 14/11/2014 (Approval letter number: 002/02/IAEC/11/2014/SBMCH). The experimental animals were divided into eleven groups and each group consisted of six animals.
Drugs and Chemicals
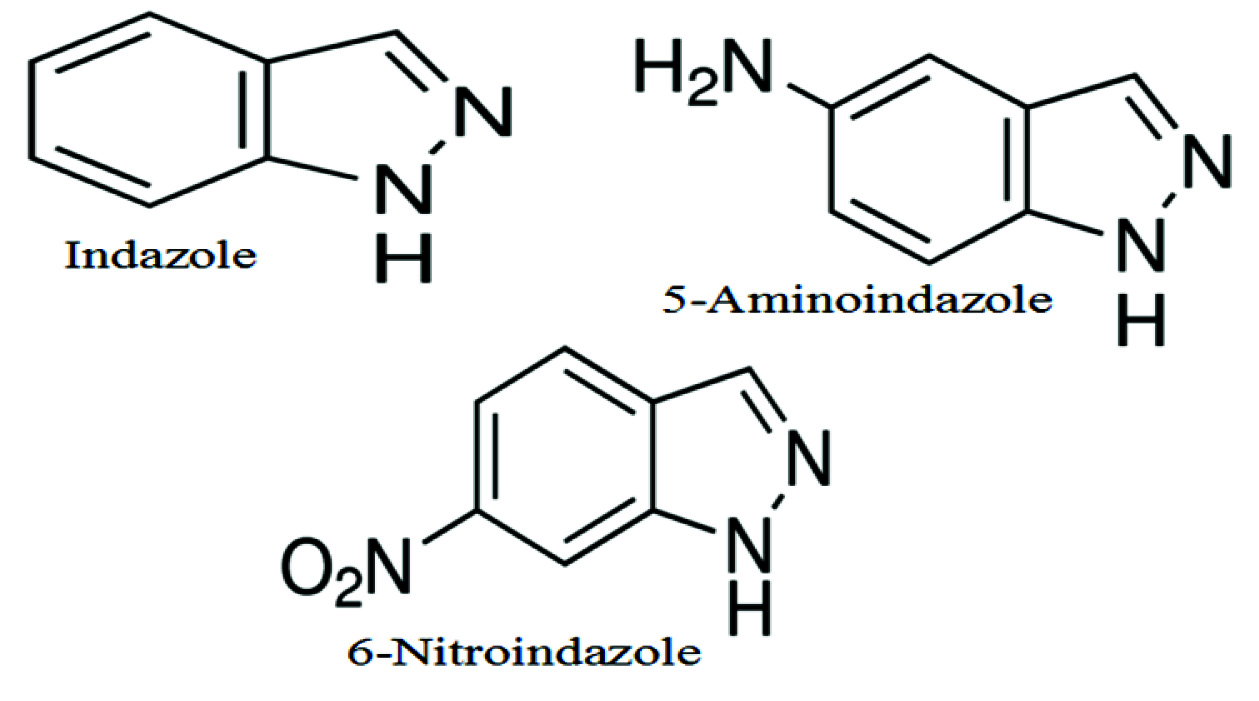
After careful scrutiny of the literature, indazole, 5–aminoindazole and 6 –nitroindazole were selected for the study [Table/Fig-1] and these were purchased from Sigma Aldrich, USA. The test compounds were prepared as a fine suspension in 0.5% Vehicle - Carboxy Methyl Cellulose (CMC) and injected i.p in doses ranging from 25-100 mg/kg, 30 min prior to the test procedures. Carrageenan (Sigma Chemical Co., USA) was used as a phlogistic agent and diclofenac sodium (Novartis, Chennai) was used as a standard anti-inflammatory agent. In vitro assay of cyclooxygenase-2, Interleukin-1β and Tumor necrosis factor–α were performed by using diagnostic kits (Cayman, USA).
Structure of indazole and its derivatives.
Carrageenan Induced Hind Paw Oedema in Rats [
14]
The animals were divided into 11 groups.
Group I - Control group (Carboxy methyl cellulose – CMC)
Groups II - Standard group (Diclofenac 10mg/kg)
Group III - Indazole – 25mg/kg
Group IV - Indazole – 50mg/kg
Group V - Indazole – 100mg/kg
Group VI - 5 Aminoindazole – 25mg/kg
Group VII - 5 Aminoindazole – 50mg/kg
Group VIII - 5 Aminoindazole – 100mg/kg
Group IX - 6 Nitroindazole – 25mg/kg
Group X - 6 Nitroindazole – 50mg/kg
Group XI - 6 Nitroindazole – 100mg/kg
Oedema was induced in Wistar male rats by injecting carrageenan (0.1 ml of 1% w/v solution) subcutaneously into the sub-plantar surface of the right hind paw. Indazole and its derivatives were administered in doses of 25, 50 and 100 mg/kg, intraperitoneally to different group of animals 30 min prior to carrageenan injection. Diclofenac (10mg/kg, intraperitoneally) was used as a standard anti-inflammatory drug. The diameter of paw was measured by using plethysmograph before administration of carrageenan and at 1, 2, 3, 4 and 5 h intervals after the administration of carrageenan. The increase in paw diameter after carrageenan injection in various indazoles and diclofenac treated animals was compared with that of vehicle (Carboxy Methyl Cellulose) treated animals. The percentage inhibition of inflammation produced by various indazoles pretreatment was calculated with standard formula (C – T / C) X 100 where C is the increase in paw diameter of vehicle treated animals and T is the increase in the paw diameter of drug treated animals.
Effect of Indazole and Its Derivatives on Cyclooxygenase – 2
Cyclooxygenase assay was carried out using COX (ovine) inhibitor screening assay (Cayman, USA). This assay directly measures PGF2α which is derived from stannous chloride reduction of PGH2 produced in the cyclooxygenase reaction. 50 μl of PGH2, indazole and its derivatives (dissolved and diluted in DMSO to 5-50μM) were incubated with reaction buffer and arachidonic acid. The reaction was allowed to proceed for precisely 2 minutes upon which the reaction was quenched by addition of 50 μl of stannous chloride solution. The reaction was allowed to proceed for an additional 10 min. Further, serial dilutions were made with EIA buffer (Available with ELISA kit) and PGF2α was measured using enzyme immuno assay (EIA). The amount of PGF2α was inversely proportional to the amount of COX-2 present in the well. Celecoxib was used as standard COX-2 inhibitors.
The percentage cyclooxygenase inhibition was calculated from the following formula:
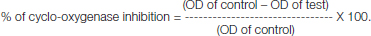
Effect of Indazole and Its Derivatives on Interleukin-1β (IL-1β) and Tumour Necrosis Factor (TNF- α)
Freshly heparinised human whole blood was used for this immunometric assay. This assay is based on a double antibody “sandwich” technique. Microwell plate supplied with the commercial kit (Cayman USA) has been coated with monoclonal antibody specific for TNF-α or IL-1β. They will capture any TNF-α or IL-1β introduced in the well. Various concentrations of indazole and its derivatives (dissolved and diluted in DMSO to 25-250μM) were added to the well. A 50 μl of acetylcholinesterase (AChE), which binds selectively to a different epitope on the TNF-α or IL-1β molecule, was also added to the well. When TNF-α or IL-1β (standard or sample) was added to the well, the two antibodies form a sandwich by binding on opposite sides of the TNF-α or IL-1β molecule. The concentration of the analyte was then determined by measuring the enzymatic activity of the acetylcholinesterase by adding Ellman’s reagent (which contains the substrate for AchE) to each well. The product of the AchE- catalysed reaction has a distinct yellow colour which shows strong absorbance at 412 nm. The intensity of this colour, determined spectrophotometrically is directly proportional to the amount of bound conjugate which in turn is proportional to the concentration of the TNF-α or IL-1β. Dexamethasone was used as a standard for TNF-α and IL-1β assay. The percentage cytokine inhibition was calculated from the following formula
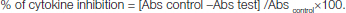
In vitro lipid Peroxidation Inhibition Assay [
15]
A 10% liver homogenate (chicken liver purchased from local butcher shop) was homogenized with polytron in ice cold Tris-HCl (20mM) buffer (pH 7.4). The homogenate was centrifuged at 3000 rpm for 10 minutes at 4°C and the supernatant fluid was then removed and used for the lipid peroxidation assay. The incubation mixture contained 1ml supernatant of liver homogenate, 0.5 ml ferrous sulphate (10mM), 0.5 ml Ascorbic Acid (0.1mM) and various concentrations of indazole and its derivatives (1-200μg/ml) dissolved in ethanol (the final ethanol concentration was 0.1% (v/v) and left at 37°C for 1 h to induce lipid peroxidation. The reaction was stopped by the addition of 1.0 ml trichloroaceticacid (TCA, 28%, w/v) and 1.5ml thiobarbituric acid (TBA, 1%, w/v). The solution was heated at 100°C for 15 minutes then centrifuged to remove the precipitated protein. Control experiments without test compounds were conducted in an identical manner. The lipid peroxidation was evaluated by determining the formation of Malonyldialdehyde (MDA), the main decomposition product of lipid peroxidation to yield a stable chromophore which is measured at 532 nm.
The percentage inhibition was calculated using the formula:
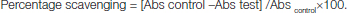
DPPH Scavenging Activity [
16]
2, 2 Diphenyl-1-Picryl Hydrazyl (DPPH) is a stable free radical, showing a deep violet colour, characterized by an absorption band in ethanol solution at 517nm. When a solution of DPPH is mixed with that of a substance that can donate a hydrogen atom, the free radical DPPH is reduced to corresponding hydrazine. Stock solution of DPPH was prepared by dissolving 25mg of DPPH in 100ml of ethanol. 2ml of reaction mixtures containing 1.9ml of DPPH and 0.1 ml of indazole and its derivatives of various concentrations (1 - 200μg/ml) dissolved in ethanol were prepared. Control reaction mixture without the test compound was prepared in an identical manner. The reaction was allowed to be complete in the dark for about 20 min. Then the absorbance of test mixture was read at 517nm. The activity was compared with Vit E (1- 200μg/ml), which was used as a standard antioxidant.
The percentage DPPH inhibition was calculated from the following formula

Nitrogen Derived Radical Scavenging Activity [
17]
This assay is based on the principle that sodium nitroprusside in aqueous solution at physiological pH spontaneously generates nitric oxide, which interacts with oxygen to produce nitrite ions. Aqueous solution of sodium nitroprusside spontaneously generates nitric oxide (NO) at physiological pH, which interacts with oxygen to produce nitrate ions which can be measured colorimetrically. 3ml of reaction mixture containing 2ml of sodium nitroprusside in phosphate buffered saline and 1ml of various concentrations (1.95, 3.90, 7.80, 15.62, 31.25, 62.5,125 and 250μg/ml) of the indazole and its derivatives dissolved in ethanol were incubated at 37oC for 5 hours. Control without test compound was kept in an identical manner. After incubation, 0.5 ml of Griess reagent (100ml of 1.0% sulfanilamide (prepared in 3M HCL) and 100 ml of 0.1% N-naphthylethylene diamine) was added. The absorbance of the chromophore formed was read at 546nm. The percentage inhibition of nitric oxide generation was measured by comparing the absorbance values of control and test compound. A standard antioxidant Vit E (1-250μg/ml) was used for comparison.
The formula used for calculation was
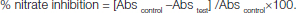
Statistical Analysis
The results were subjected to ANOVA and Dunnet’s t-test (SPSS version 16.0 software was used). A p-value less than 0.05 were considered statistically significant. For in-vitro assays a mean of three observations was represented. The IC50 values of indazole and its derivatives for inhibition of COX - 2, TNF- α and IL-1β were obtained by linear regression analysis.
Results
In vehicle treated animals (CMC), carrageenan administration in the hind paw resulted in progressive increase in the paw diameter and a maximum of 3.20 cm was recorded at second hour. In diclofenac (10 mg/kg) pretreated animals 47.21% inhibition of inflammation was noted in the first hour, which increased gradually in the remaining observation periods and reached to 84.50% at the end of fifth hour [Table/Fig-2]. A time dependent and dose dependent inhibition of paw oedema was observed after treatment with different doses of indazole and its derivatives.
Effect of Indazole on rat paw oedema. Each value represents the mean ± SEM of six observations.
Indazole treatment significantly attenuated the increase in paw diameter after carrageenan administration in a dose dependant fashion compared to vehicle treated animals. A maximum of 61.03% inhibition of inflammation was recorded for 100 mg/kg of indazole during the fifth hour of observation compared to 84.50% recorded for diclofenac [Table/Fig-2].
Pre-treatment with 5–aminoindazole resulted in 15.87 ± 0.21, 19.31 ± 0.46 and 34.33 ± 0.51 percent inhibition of inflammation was observed in 25mg/kg, 50mg/kg and 100mg/kg respectively in the first hour. In similar doses, a maximum of 39.90 ± 0.36, 60.09 ± 0.32 and 83.09 ± 0.41 percent inhibition of inflammation was noted in the fifth hour for 5 – aminoindazole [Table/Fig-3]. The percentage inhibition of 5 - aminoindazole in a dose of 100 mg/kg could offer 83% inhibition of paw oedema during the fifth hour which is almost comparable to the effect of diclofenac (84%).
Effect of 5 - Aminoindazole on rat paw oedema. Each value represents the mean ± SEM of six observations.
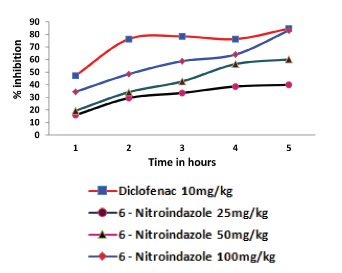
A similar pattern of response was noted for 6 – nitroindazole. In the fifth hour, 6 – nitroindazole produced a maximum of 37.55 ± 0.41, 52.58 ± 0.46 and 71.36 ± 0.56 percent inhibition of inflammation in 25mg/kg, 50mg/kg and 100mg/kg respectively [Table/Fig-4].
Effect of 6 - Nitroindazole on rat paw oedema. Each value represents the mean ± SEM of six observations.
Effect of Indazole and Its Derivatives on Cyclooxygenase -2
A concentration dependent inhibition of cyclooxygenase–2 was clearly evident for all the tested indazoles. A maximum of 78% inhibition was recorded for 5–aminoindazole in a concentration of 50 μM. In the same concentration maximum inhibition of 70% and 68% was recorded for indazole and 6–nitroindazole respectively [Table/Fig-5]. The IC50 values of indazole, 5-aminoindazole and 6 - nitroindazole were 23.42, 12.32 and 19.22 μM respectively. The IC50 value for celecoxib in the same assay procedure was found to be 5.10 μM.
Effect of indazoles on Cyclooxygenase - 2 (COX – 2). Each value represents the mean of three observations
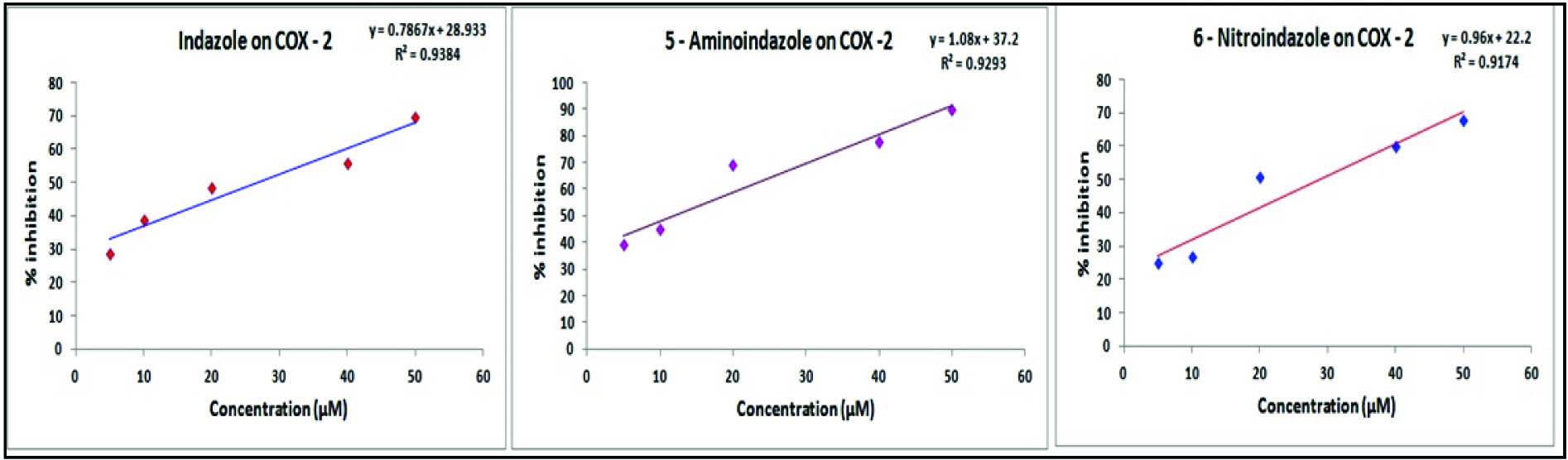
Effect of indazole and its derivatives on TNFα: The investigated indazoles inhibited TNF-α in a concentration dependent manner. A fairly high degree of inhibition was recorded for indazole and 5 - aminoindazole in concentrations ranging from 25 – 250 μM [Table/Fig-6]. A maximum of 62% and 58% inhibition of TNF-α was recorded for 250μM concentration of indazole and 5–aminoindazole respectively [Table/Fig-6]. The TNF – α inhibitory activity (29%) of 6 - nitroindazole was the least among the tested compounds. Dexamethasone used as a standard drug exhibited an IC50 value of 31.67μM, while that of other compounds were indazole - 220.11 μM, 5-aminoindazole -230.19μM. IC50 value of 6 – nitroindazole was not calculated because it produced only 29% inhibition of TNF – α in the maximum concentration (250 μM) employed in assay.
Effect of indazoles on TNF-β. Each value represents the mean of three observations.
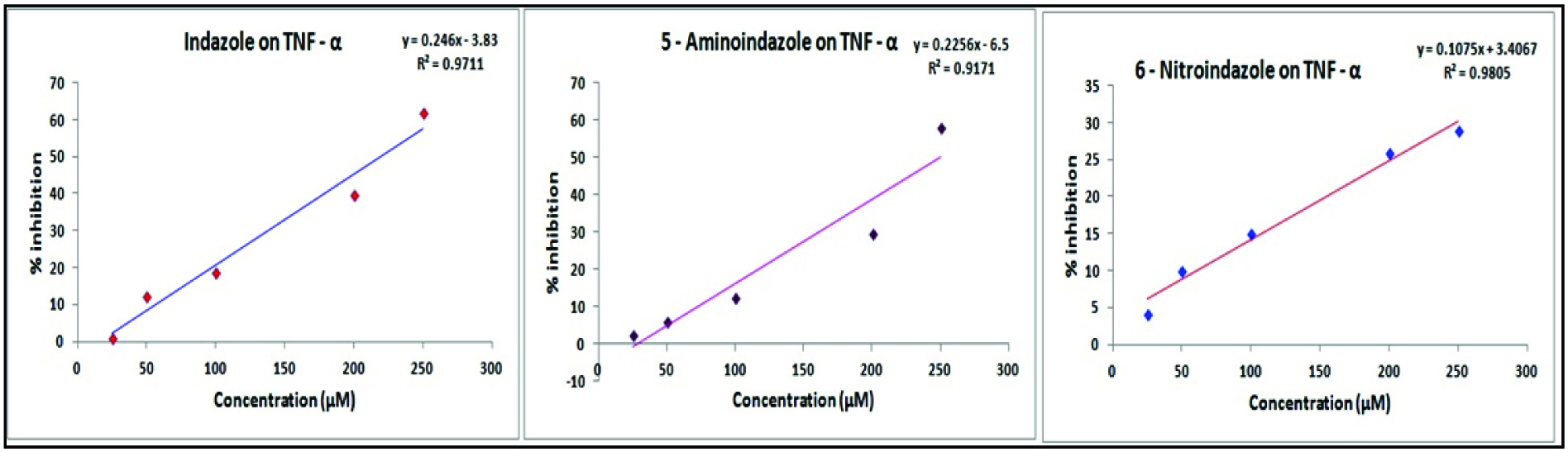
Effect of indazole and its derivatives on - 1β (IL-1β): A concentration dependent inhibition of IL - 1β activity was recorded for all the tested indazoles. More than 70% inhibition was recorded for all the investigated indazoles in a concentration of 250 μM. [Table/Fig-7]. The IC50 values of indazole, 5 – aminoindazole and 6 - nitroindazole were 120.59 μM, 220.46 μM and 100.75 μM respectively. The IC50 value of dexamethasone to inhibit interleukin 1β in the same assay procedure was found to be 102.23 μM. It can be appreciated that the IC50 value of 6 – nitroindazole is slightly lower to that of dexamethasone, whereas the other compounds possessed a higher IC50 value.
Effect of indazoles on IL-1β. Each value represents the mean of three.
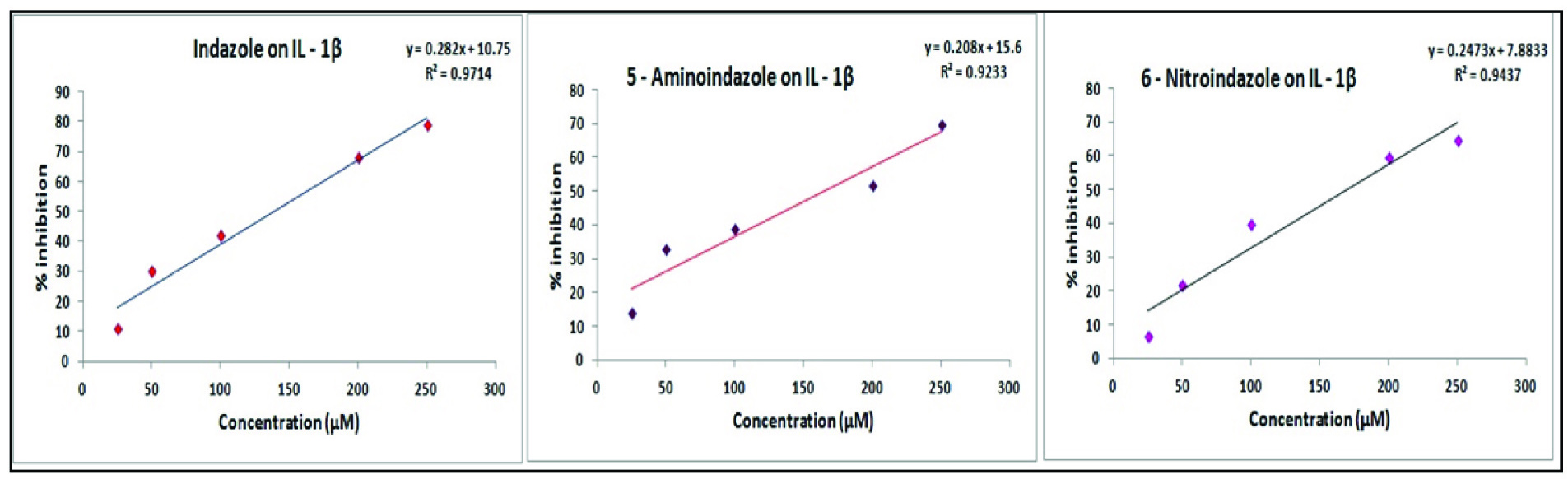
Effect of indazole and its derivatives on lipid peroxidation: A concentration proportionate inhibition of lipid peroxidation was evident for the investigated indazole and its derivatives [Table/Fig-8]. In a concentration of 1μg/ml, indazole, 5 – aminoindazole and 6–nitroindazole inhibited lipid peroxidation to an extent of 26.44, 16.53 and 25 percent respectively. The above compounds when employed in a concentration of 200μg/ml produced 64.42, 81.25 and 78.75 percent inhibition respectively. Among the tested compounds 5–aminoindazole and 6 – nitroindazole produced a higher degree of inhibition of lipid peroxidation.
Effect of indazoles on lipid peroxidation. Each value represents the mean of three observations.
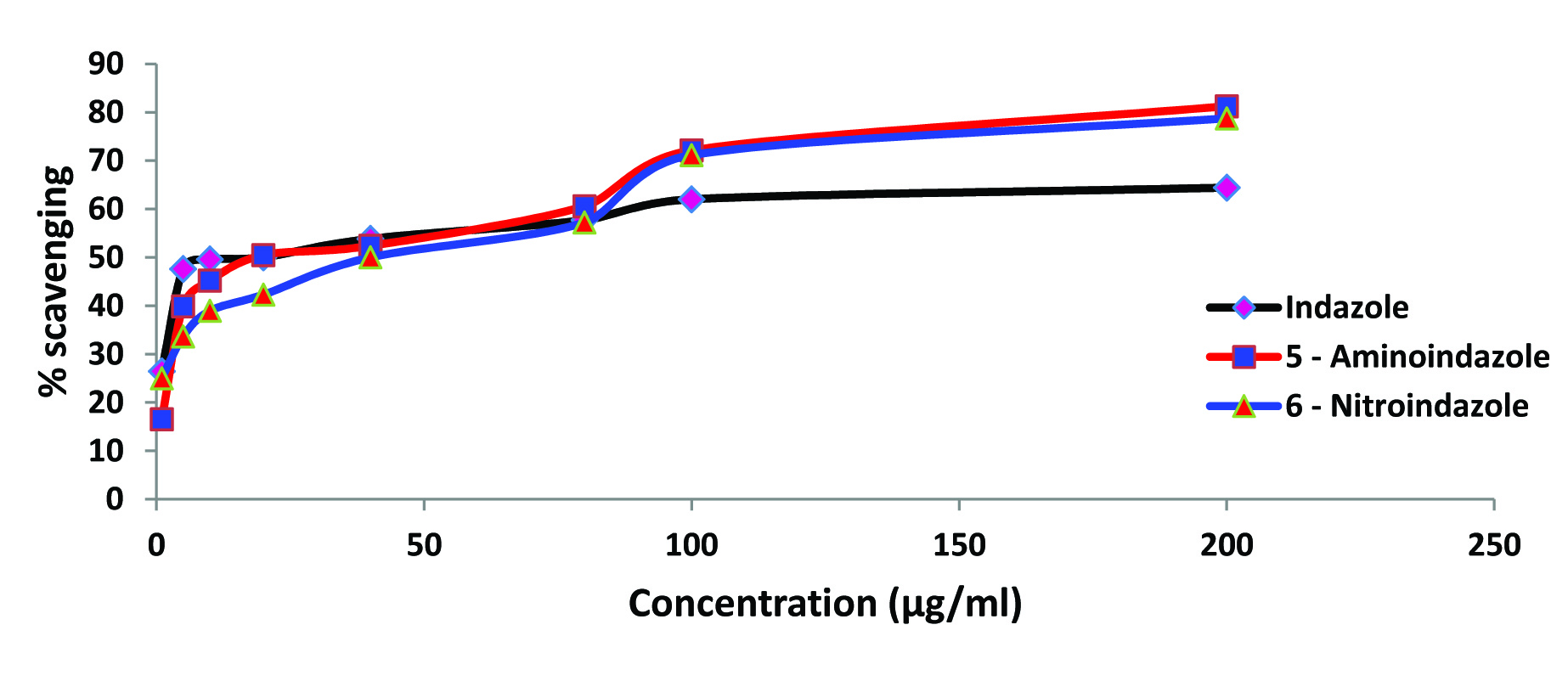
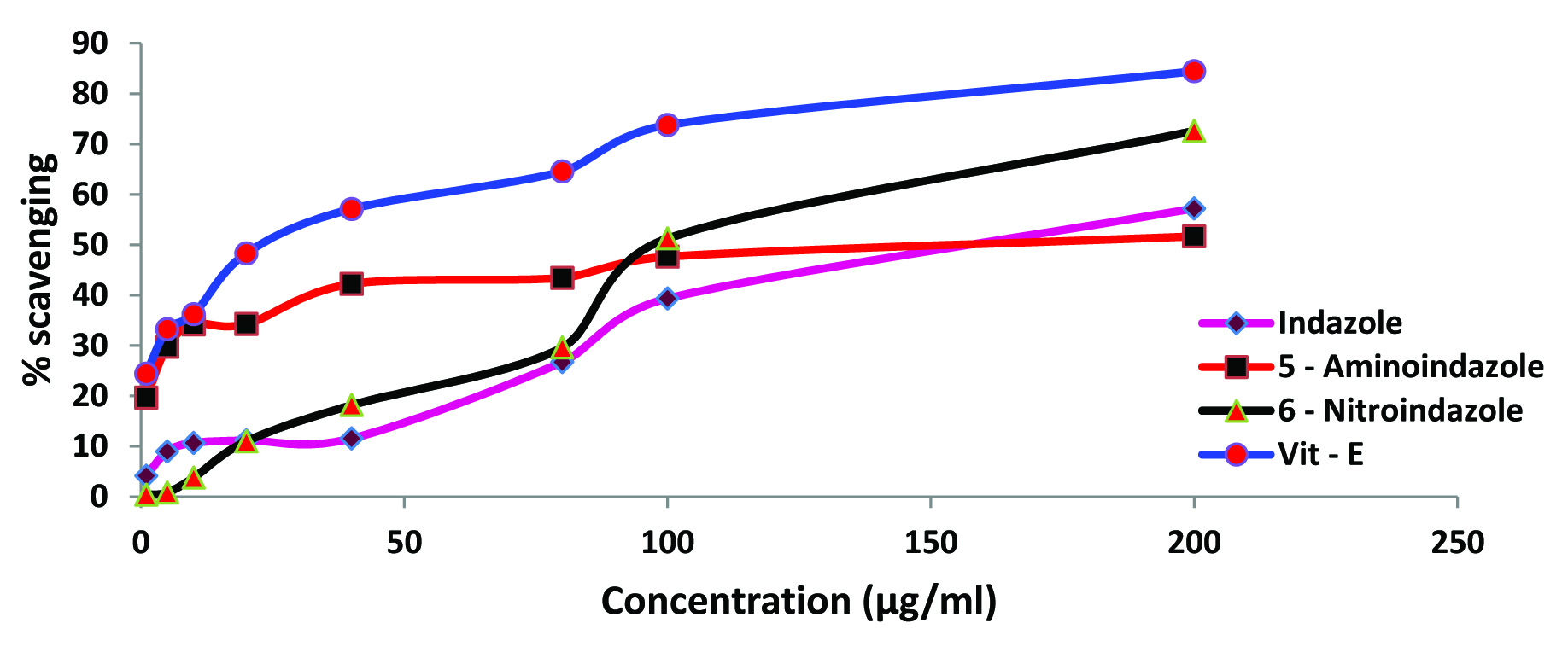
Effect of indazole and its derivatives on DPPH activity: Standard antioxidant vitamin–E produced a concentration dependent inhibition of DPPH activity ranging between 24.40 and 84.43 %. All the three tested indazoles inhibited DPPH activity in a concentration depended manner [Table/Fig-9]. A maximum of 51.21% and 57.21% inhibition was recorded for 5 – aminoindazole and indazole respectively in a concentration of 200μg/ml. A higher degree of inhibition (72.60%) was evident for the 6 – nitroindazole in the same concentration.
Effect of indazoles on DPPH scavenging. Each value represents the mean of three observations.
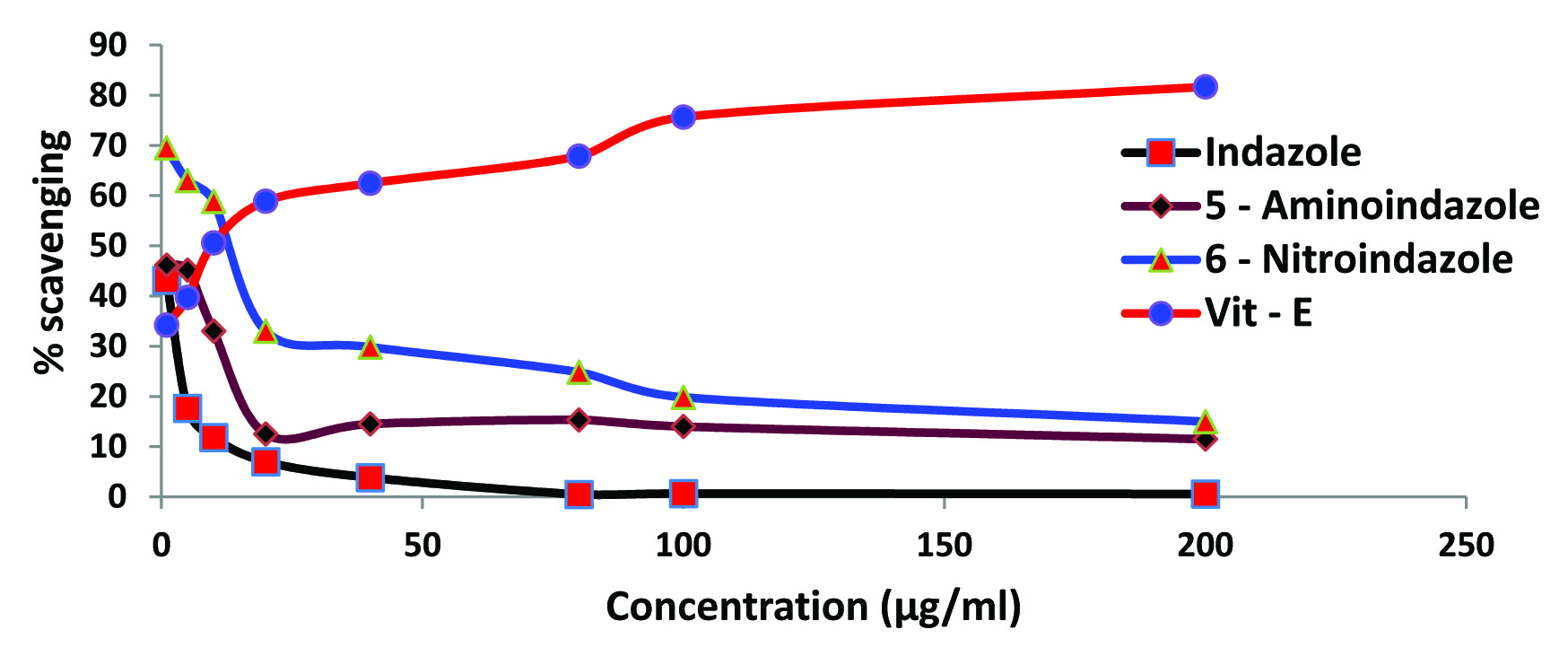
Effect of indazole and its derivatives on nitrite ion generation: Vitamin–E was found to produce a concentration dependent inhibition of nitrite ion generation. A maximum of 81.67% inhibition was recorded for vitamin-E in a concentration of 200μg/ml. Investigated indazoles exhibited a varying degree inhibition of nitrite ion generation. In a concentration of 1μg/ml, an inhibition of 43.16, 46.16 and 69.5 percent were recorded for indazole, 5 – aminoindazole and 6 – nitroindazole respectively. However, in the maximum concentration (200μg/ml) the generation of nitrite ion inhibitory activity was drastically reduced in all the investigated compounds [Table/Fig-10]. In the highest concentration (200 μg/ ml), all the indazoles showed an inhibition ranging from 0.58 – 15% compared to 81.67% observed with vitamin–E.
Effect of indazoles on nitric oxide (NO) scavenging. Each value represents the mean of three observations.
Discussion
Indazole and derivatives were screened for their effect on acute inflammation by using a well established animal model of carrageenan induced hind paw oedema in rats. Carrageenan is a phlogistic agent has been widely used to induce experimental inflammation for the screening of compounds possessing anti - inflammatory activity [18]. When carrageenan is injected locally in to the rat paw it produces a severe inflammatory reaction, which is noticeable within 30min [19]. The development of oedema induced by carrageenan is a biphasic event; the early phase event (60 – 120 min) of the inflammation is due to the release of histamine, serotonin and similar substances. The later phase (120 – 300 min) is associated with the activation of kinin – like substances and the release of prostaglandins, proteases and lysosome [20]. In the present study, all the investigated indazoles showed a significant, dose dependent and time dependent inhibition of carrageenan induced hind paw oedema [Table/Fig-2,3 and 4]. Consistent reduction in paw oedema was noticeable for all the tested compounds from the second hour of carrageenan administration. The maximum reduction of paw oedema was observed at fifth hour in a dose of 100mg/kg for investigated indazoles (61.03 – 83.09%). The compound 5–aminoindazole produced a maximum inhibition of 83.09% in 100 mg/kg dose. Diclofenac treated animals showed noticeable reduction of paw oedema in all the observation periods, which was more obvious from the second hour observation [Table/Fig-2]. Thus, the present study reveals the anti–inflammatory potential of investigated indazole and its derivatives in addition to their antinociceptive activity reported earlier [13].
Several endogenous inflammatory mediators such as prostaglandins, cytokines and free radicals are involved in not only the initiation but also in the perpetuation of inflammation. In the present study, the effect of indazoles on some of these mediators of inflammation has been investigated.
Cyclooxygenase (COX) is an important enzyme system in the cascade of inflammatory reaction. The cyclooxygenase enzyme exists in a constitutive (COX – 1) and inducible (COX – 2) isoforms. The COX – 1 serves physiological housekeeping functions, while COX – 2 normally present in insignificant amounts, is inducible by cytokines, growth factors and other stimuli during inflammatory response [21]. Most NSAID inhibit both COX – 1 and COX – 2 with little selectivity. The results of the present study reveal that the investigated compounds inhibit the cyclooxygenase COX – 2 to varying degree [Table/Fig-5]. The inhibition of COX – 2 ranged from 68 to 78% for the various indazoles in the maximum concentration (50μm). Among the tested compounds, 5–aminoindazole exhibited a maximum activity in inhibiting COX-2 in all the concentrations employed in this assay [Table/Fig-5]. The IC50 of different indazoles to inhibit COX – 2 ranged from 12.32 – 23.42 μM. It is obvious from the results that, the investigated indazoles exerted a significant inhibitory action on cyclooxygenase. This observation indicates that cyclooxygenase inhibition may be one of the important mechanisms for the antiinflammatory action of investigated indazoles.
TNF-α is a proinflammatory cytokine mainly released from monocytes, macrophages, T-lymphocytes, neurons and glia in various pathological conditions such as Crohn’s disease, rheumatoid arthiritis, ankylosing spondylitis and psoriatic arthritis [22,23]. Monoclonal antibodies like infliximab and etanercept directed against TNF-α were used in treating rheumatoid arthiritis and ankylosing spondylitis [24]. TNF-α mainly acts by two cell surface receptors TNFR1 and TNFR2, which are up regulated during inflammation [25]. The main biological function of TNF– α is to induce inflammation via the upregulation of gene transcription, primarily through the nuclear factor – kappa beta (NFkβ) and Activator protein – 1 (AP -1) signalling pathways [26]. Indazole and its derivatives showed a concentration dependent inhibitory activity on TNF– α [Table/Fig-6]. In a maximum concentration 250 μM more than 60% inhibition of TNF – α was achieved by indazole. However, the TNF–α inhibitory activity of 6 - nitroindazole was only 29% even with the maximum concentration of compound employed (250 μM), so the IC50 could not be calculated. Dexamethasone used as a standard drug exhibited an IC50 value of 31.67 μM, while that of other compounds were indazole - 220.11 μM, 5 - aminoindazole - 230.19 μM.
IL-1 β is a proinflammatory cytokine that exerts pleiotropic effects on a variety of cells and plays key roles in inflammation, immunity and haemopoiesis [27]. However, over production of IL-1β is implicated in the pathophysiological changes that occur during various disease states, such as rheumatoid arthritis, inflammatory bowel disease, osteoarthritis, vascular disease, multiple sclerosis, and Alzheimer’s disease [28–30]. The carrageenan induced mechanical or thermal hyperalgesia was associated with an upregulation of IL-1β [31]. Results of the present study revealed a potent inhibitory activity of indazole and its derivatives on IL-1β [Table/Fig-7]. The maximum inhibition of IL-1β for indazole and its derivatives ranged from 73% to 79%. The IC50 value of 6 – nitroindazole (100.75 μM) was slightly lesser than the IC50 value of standard drug dexamethasone (102.23 μM).
Free radicals are derivatives of molecular oxygen and nitrogen and consist of superoxide, hydroxyl radical, hydrogen peroxide and peroxynitrite. They are normally removed by antioxidant systems including superoxide dismutase, catalase, glutathione, glutathione peroxidase, α-tocopherol and vitamin –C [32,33]. Thus, their levels are precisely controlled by antioxidant systems. However, in pathologic conditions, levels of free radicals may increase due to the increased production or decreased antioxidants level [34]. Overproduction of Reactive Oxygen Species (ROS) and other related radicals lead to oxidative stress which plays a significant role in arthritis or other inflammatory disorders. During arthritis large quantity of superoxide and hydrogen peroxide radicals are generated due to massive accumulation of granulocytes and macrophages [35]. The reactive oxygen species generated from activated phagocytes have been implicated in inflammation and tissue destruction [36]. In rheumatoid arthritis, many drugs act by reducing oxidative damage at inflammatory sites either by inhibiting reactive oxygen production by phagocytes or by scavenging such noxious radicals [37]. Based on the above information it was considered interesting to investigate the effect of these compounds on free radical scavenging activity against lipid peroxidation, DPPH and nitric oxide.
Lipid peroxidation is an oxidation reaction in which free radicals generated against lipids [38], resulting in disturbance of membrane phospholipids assembly which lead to permeability of lipid bilayers, alterations of ion transport, inhibition of metabolic processes and further generation of reactive oxygen species due to damage of mitochondria [39–41]. In the present study, lipid peroxidation was inhibited by 5 – aminoindazole and 6 – nitroindazole to an extent of 70% and the other investigated compound produced a lesser degree of inhibition [Table/Fig-8].
Various concentrations of indazole and its derivatives inhibited DPPH free radical generation in a concentration dependent manner [Table/Fig-9]. A maximum inhibition of DPPH radical generation for the test compounds ranged between 39.35% and 51.26%. Among the tested compounds, 6 – nitroindazole exhibited highest inhibition of DPPH free radical generation. The free radical scavenging activity was further corroborated by the ability of the tested indazoles in scavenging nitric oxide. However, nitric oxide scavenging activity was only observed in low concentrations of indazole and its derivatives but not in high concentrations [Table/Fig-10].
Limitation
1) Study could further extended to other spices also.
2) Only acute anti- inflammatory model was used.
Conclusion
In conclusion, the findings of the present study have identified indazole and its derivatives with marked anti-inflammatory activity. The anti-inflammatory activity of investigated indazoles may be through mechanisms that involve an interaction with cyclooxygenase - 2, cytokines and reactive oxygen species.