In an earlier study we had shown that purified 35-kDa HAP from TSB culture supernatant can accumulate haemorrhagic fluid in the rabbit ileal loop and histopathological studies demonstrated the presence of red blood cells, neutrophils and eosinophils [16]. Our data suggested that HAP might play an important role in the inflammatory response in cholera. In this work our purpose was to identify the causative agents of V. cholerae mediated extra-intestinal infection.
Materials and Methods
Animal experiments were done (during April to August 2007) after obtaining necessary permission from Institutional Animal Ethical Committee (IAEC) of National Institute of Cholera and Enteric Diseases, Calcutta, India.
Bacterial strains, plasmids and growth conditions: The strain C6709 was selected for the study since it carries a functional hapR gene, the product of which regulates several cellular processes in Vibrio cholerae including expression of HAP [19]. Vibrio cholerae (C6709) cells were routinely grown in Luria broth (LB) at 37oC with shaking for 18 hours [20]. However, V. cholerae cells were also grown in Tryptic Soy Broth (TSB) (Difco, USA) for various experimental purposes as described below. Bacterial cells were maintained at –70oC in LB containing 20% sterile glycerol [19].
Purification of HAP after removal of Outer Membrane Vesicles (OMVs): The V. cholerae O1 strain C6709 was grown and harvested as described above. The cell-free culture supernatant was sequentially filtrated through 0.45-μm and 0.22-μm-pore size cellulose acetate membranes (millipore) to remove residual cells. The OMVs were removed from the resulting filtrates by centrifugation at 150,000 x g for 3 h at 4°C using a T-865 rotor (Sorval Instruments, USA) [10]. The supernatant was carefully aspirated from ultracentrifuge tubes followed by salting out with 60% saturated ammonium sulfate. Protein was precipitated by centrifugation at 10,000 rpm for 20 minutes at 4OC. Precipitated protein was resuspended in 25mM Tris-HCl buffer pH 7.4, followed by dialysis against same buffer and concentrated with an Amicon filtration (Milipore, usa), purified by a single step ion-exchange chromatography (DE-52) which was pre-equilibrated with 25mM Tris-HCl buffer (pH 7.4). Proteins were eluted in the unbound fractions and constituted distinct peaks, pooled, dialyzed, concentrated and examined for haemagglutinin, protease and aminopeptidase activities. Purification of HAP by both the methods was carried out essentially in absence of EDTA. The columns were run in Biologic Duo Flow Chromatographic System (Bio-Rad, USA).
Sodium Dodecyl Sulfate-Polyacrylamide Gel Electrophoresis (SDS-PAGE). SDSPAGE was performed by the method of Laemmli [21]. Protein samples after TCA precipitation were denatured in sample buffer and resolved in a 12.5% polyacrylamide gel with a discontinuous buffer system.
Protease assay. In this study azocasein was chosen as a substrate to assay the proteolytic activity of HAP [16]. The substrate-enzyme (azocasein-HAP) mixture was incubated at 37°C for 1 hour followed by termination of the reaction with 10% (w/v) trichloroacetic acid. The precipitated protein was removed by centrifugation (12,000 rpm for 4 min), and the supernatant was transferred to a clean tube containing 525mM NaOH. Absorbance was measured at 440nm using a Shimadzu spectrophotometer (Shimadzu, Japan). Substrate with buffer and substrate with the heat-inactivated enzyme were used as negative controls.
Haemagglutinin assay. The technique for quantification of haemagglutinin activity was adopted from Jones et al., [22]. Briefly, protein samples were serially diluted (1:2) in a micro titer plate and an equal volume of 1% rabbit red blood cells were added to each well and incubated at room temperature for 60 min. Purified HA from a V. cholerae serogroup O2 strain was obtained [23]. Purified HA was used as a positive control and 25mM Tris-HCl buffer, pH 7.4 was used as negative controls.
Leucyl-p-nitroanilide assay. The aminopeptidase activity was measured to detect the presence of Leucine Aminopeptidase (LAP) by a leucyl-p-nitroanilide assay as described previously by Prescott and Wilkes [24]. The increase in A405 due to liberation of p-nitroaniline was measured spectrophotometrically. To study the enzyme activity sample solution was pre-incubated with EDTA (10Mm) or Bestatin (100 μM) for 30 minutes at 37OC.
An 8 μl of the sample was added to 2 ml of substrate followed by observing the increase in absorbance at 25OC within a period of one minute.
N-terminal amino acid sequencing. Sequencing of N-terminal amino acid residues of the 35 kDa purified HAP protein band recovered from an SDS-PAGE gel was carried out by using an automated protein sequencer (Model 491, Applied Biosystems, Tokyo, Japan). Homology search was performed using the BLAST (Basic Local Alignment Search Tool) and FASTA programs (Multistep algorithm for sequence alignment).
Raising antibody against purified HAP. Antiserum to purified HAP was prepared by immunizing a New Zealand White rabbit by intra-muscular injection with 100μg of HAP emulsified with an equal volume of Freund’s Complete adjuvant (Sigma, USA). This was followed by a booster injection with incomplete adjuvant (Sigma, USA) and two further boosters with HAP alone administered at 14-day intervals. Three days after the fourth injection, blood was drawn and serum was collected and stored at -20°C. The serum was collected and antibody titer was determined by Enzyme-Linked Immunosorbent Assay (ELISA) with the pre-immune sera as the control [25].
Immunoblotting. The proteins were separated in by SDS-PAGE and transferred to nitrocellulose membrane (pore size 0.45μm Bio-Rad) electrophoretically [26] with a transblot apparatus (Bio-Rad, USA). The Nitrocellulose sheet was subsequently washed and incubated at 37°C with polyclonal antibody against HAP (1:1000 dilution {v/v}) and goat anti-rabbit immunoglobulin G (Jackson Immuno Research laboratories, Inc.) conjugated to alkaline phosphatise (1:2,000 dilution [v/v]). Colour was developed by 5-bromo-4-chloro-3-indolylphosphate and nitroblue tetrazolium solution (Bio-Rad).
Subcutaneous mouse assay. Subcutaneous mouse assay (4 to 5 day old suckling mouse) was performed with modifications of a previously described method [17] with different doses (1, 125 ng; 2, 250 ng; 3, 500 ng; 4, 1 μg; 5, 2 μg; 6, 4 μg and 7, 8 μg) of purified V. cholerae HAP. Each sample was injected carefully under lower dorsal side of skin of a suckling mouse using a 26-gauge needle (BD Diagnostics, India). Similar in vivo assay was carried out with 0.1 ml of the TSB media or culture supernatant of the wild-type V. cholerae strain C6709, which was prepared from overnight grown bacterial cells in TSB. Treated mice were observed for lesions, if any, and the data were recorded accordingly, after that the animals were sacrificed. Each sample was tested at least in 3 mice.
Histopathology of mouse skin. Skin specimens from in and around of the inoculation sites were collected immediately after sacrificing the animals and fixed in buffered 10% (v/v) formalin. Formalin-fixed tissues were embedded in paraffin and cut into 4-5μm thick sections. Histological sections were stained with haematoxylin and eosin and examined with a trinocular research microscope (Leica, Germany). Extensive histopathological analysis of the treated skin of mice were done to understand the extent of tissue damage due to HAP.
Degradation of Laminin and Type IV Collagen by HAP. Laminin degradation assay was done as described earlier [27]. Briefly, purified laminin {ICN, USA; (60μg dissolved in a buffer containing 50mM Tris and 150mM NaCl, pH 7.4)} was incubated with different doses (0ng, 500ng, 1.5μg and 2.5μg) of HAP for 30 min at 37oC and the same substrate/enzyme ratio (60μg laminin and 2.5 μg HAP) was incubated for 0, 5, 15 and 30 min at 37oC. The incubation temperature used is well below the denaturing temperature (58°C) of laminin (ICN, USA). The reaction was stopped by adding an equal volume of denaturing buffer (0.125 M Tris hydrochloride (pH 6.8), 4% Sodium Dodecyl Sulphate (SDS), 20% glycerol, 10% 2 mercaptoethanol), the samples were boiled for 2 min followed by SDS-PAGE using a 12.5% polyacrylamide gel as described above. Each assay was done in triplicate. On the other hand, the collagen degradation assay was carried out as done previously [27]. Briefly, purified collagen type IV (Sigma, USA; (60μg dissolved in acetic acid)] was incubated with different doses (0 ng, 500ng and 1.5μg) of purified HAP for 30 min at 37°C. The same substrate/enzyme ratio (60μg of collagen type IV and 2.5 μg HAP) was incubated at 37°C for 0, 2 and 10 min. The reaction was stopped by adding an equal volume of denaturing buffer containing 0.125M Trishydrochloride (pH 6.8), 4% Sodium Dodecyl Sulfate (SDS), 20% glycerol, 10% 2-mercaptoethanol. The samples were then boiled for 2min and SDS-PAGE using 7.5% polyacrylamide gel was performed as described above.
Tissue culture assay. The cell rounding and cell distending effects of HAP were assayed on human large intestinal cell line Int407, which were grown in a 6 well flat-bottomed tissue culture plate (Costar, USA) containing minimum essential medium (Gibco, USA) supplemented with 10% fetal bovine serum (Gibco, USA) along with penicillin G and streptomycin sulfate (Sigma, USA) at 37oC in a CO2 incubator (Heraeus, Germany). When the cells grew to confluence in a 25ml tissue culture flask, they were harvested by trypsinization and seeded in a 6 well plate at a concentration of approximately 103 cells/ well. Cells were permitted to reach confluence by allowing them to grow for 24 h in a CO2 incubator. Samples to be tested were filter sterilized and serially diluted in serum free tissue culture medium. After careful aspiration of the medium from the plate, HAP were added to the Int407 cells and incubated for 24 h and 48 h in a CO2 incubator. Dose dependent effect with purified HAP on Int407 cells was tested in a 96 well tissue culture plate. Int407 cells were examined for morphological changes with a phase contrast inverted microscope (Model CK 40, Olympus, Japan) [16].
Results
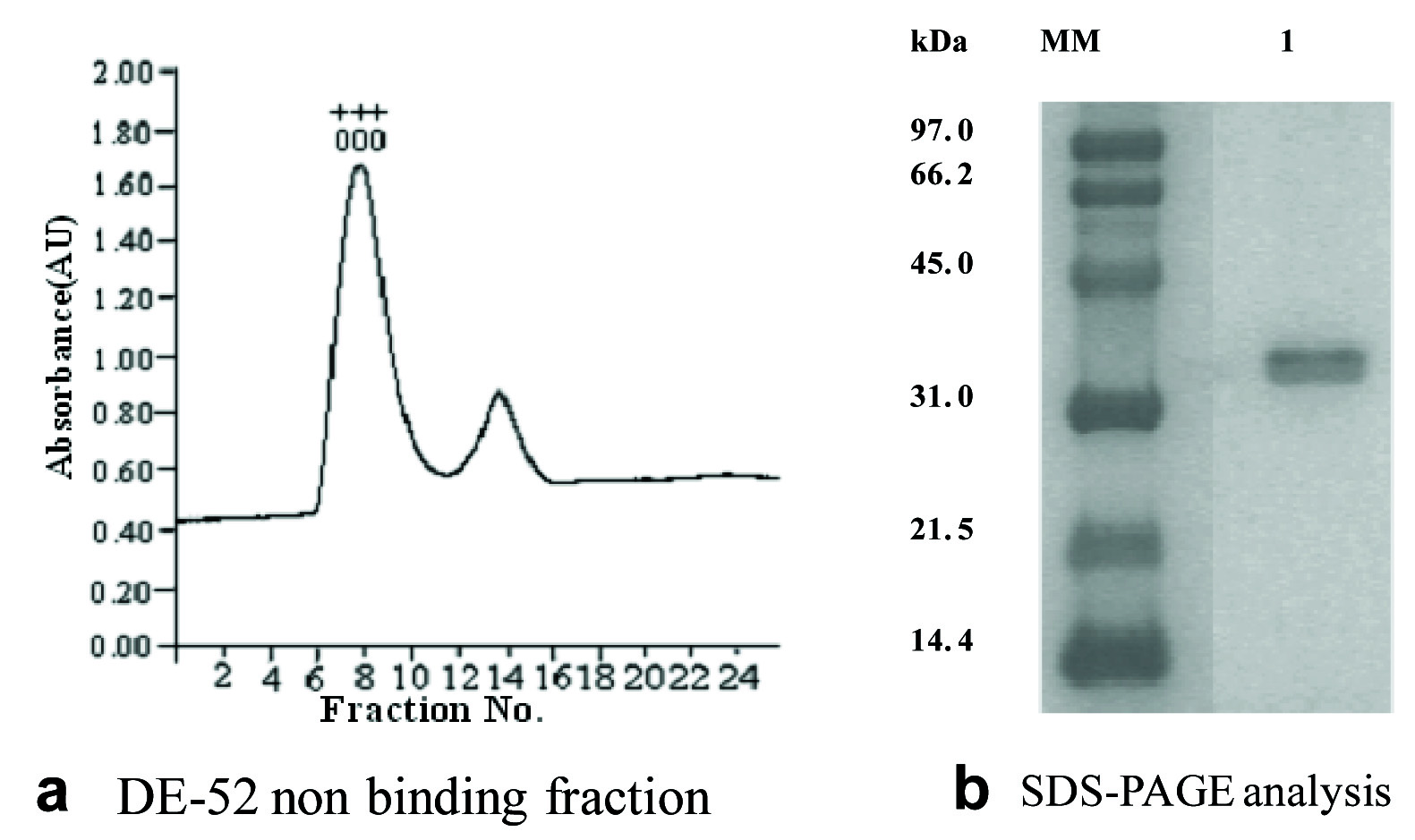
Purification of HAP in absence of OMVs. HAP was purified in the absence of OMVs [Table/Fig-1a]. A single 35-kDa band was observed on an SDS-PAGE [Table/Fig-1b]. N-terminal sequencing of protein followed by analysis with BLAST and FASTA program shows 100% homology of the initial N-terminal 15 amino acid sequence of the HAP purified from C6709 and the earlier reported sequence of HAP.
Chromatographic profiles showing purification of 35-kDa HAP from culture supernatant of V. cholerae O1 strain C6709 after removal of OMVs. a) Anion exchange chromatography on DE-52 column with ammonium sulfate precipitated proteins after removal of OMVs. Non-binding fractions showing HA and protease activities were pooled, concentrated and dialyzed. Absorbance is shown in arbitrary unit (AU). b) SDS-PAGE (12.5%) of the proteins from the single peaks shown in panels 1. The positions (in Kilodalton) of molecular mass markers (MM) are shown to the left of the gels.
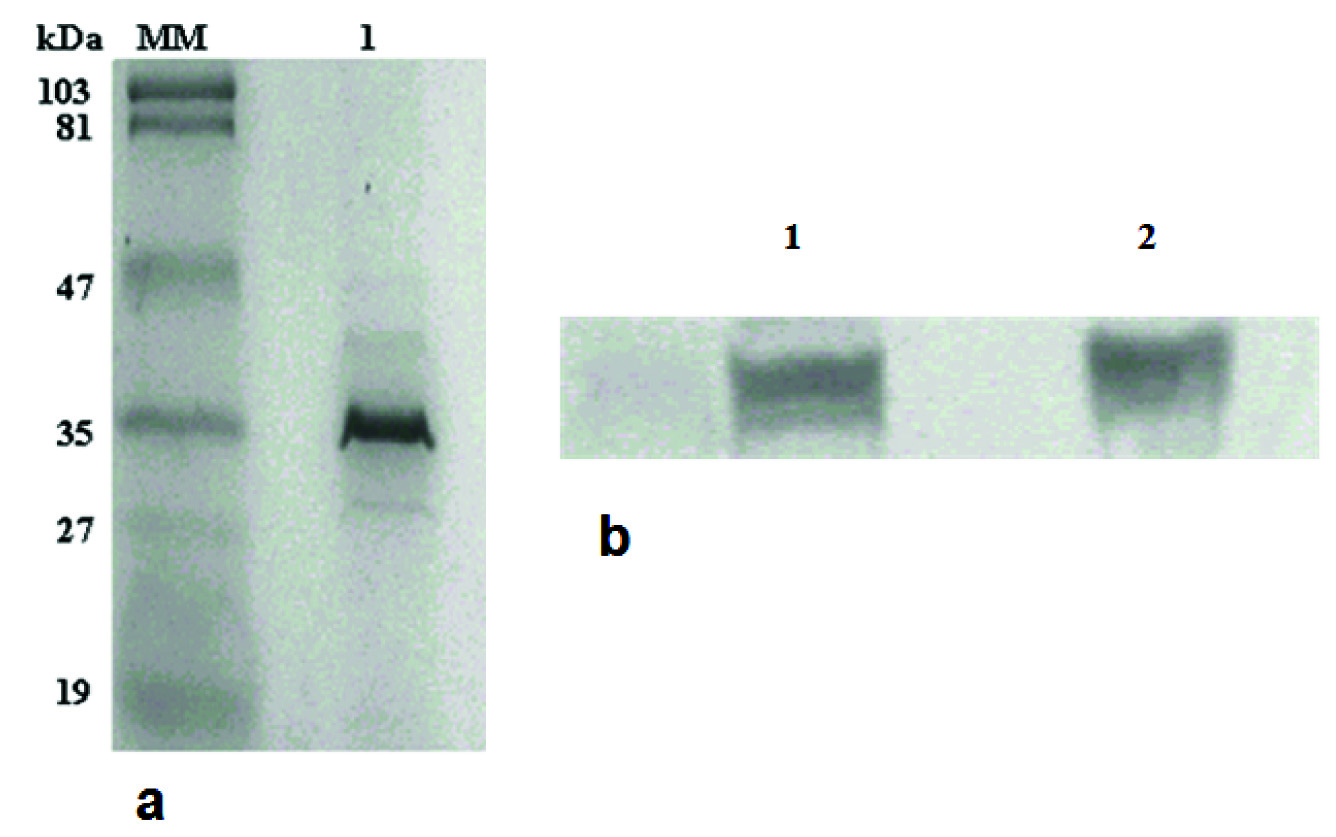
Protease assay of strain C6709. Ammonium sulfate precipitated proteins of C6709 culture supernatant showed haemagglutinin and aminopepetidase activity. SDS-PAGE analysis with ammonium sulfate precipitated proteins of C6709 showed presence of HAP in C6709 strain [Table/Fig-2a]. This was further confirmed by immunoblot analysis with the antibodies raised against the purified HAP [Table/Fig-2b]. As expected, the rabbit anti-HAP antibodies failed to detect the 35-kDa band suggesting further the deletion of the hapA gene of C6709.
(a) 12.5% SDS-PAGE of ammonium sulfate precipitated protein from the strain C6709 (lane 1). b) Western blot was performed using equal amount of ammonium sulfate precipitated proteins of C6709 (lane1) and 2.5 μg purified HAP (lane 2) with an anti-HAP antibody raised in rabbit.
Cell culture: Purified HAP from C6709 showed dose dependent cell distending and cell rounding effects on Int407 cell line [Table/Fig-3].
Effects of HAP on Int 407 Cells.
| Treatment Time (h) | Control buffer | 6μg | 3μg | 1.5μg | 750ng | 375ng | 187.5ng | 93.75ng | 46.875ng |
|---|
| 24 | Normal | CR | CR | CR | CR | CR | NR | NR | NR |
| 48 | Normal | CR | CR | CR | CR | CR | CR | CR | NR |
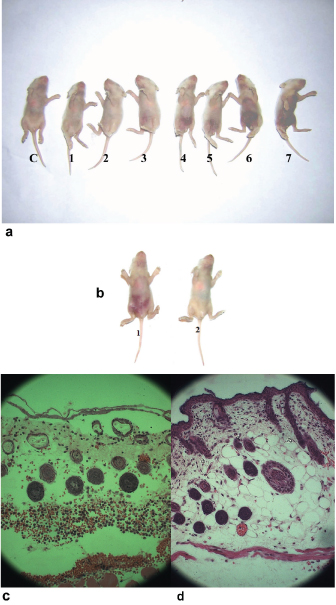
Effects of HAP on subcutaneous tissue of suckling mouse. Injection of HAP at the dorsal skin of mice showed lesion surrounding the site of the injection along with haemorrhage and oedema [Table/Fig-4a]. Lesions were observed after 1.5h of injecting the samples. The intensity of effect decreased with lower doses of HAP [Table/Fig-4a]. Mice treated with 25mM Tris-HCl (pH 7.4) as a negative control did not show any effect. Culture supernatant of C6709 strain also showed similar changes when injected under mice skin [Table/Fig-4b]. In sharp contrast, TSB medium did not show any effect in mice suggesting strongly that the haemorrhagic lesions in the mouse dorsal skin was due to HAP [Table/Fig-4b]. Histopathological changes underlying the gross pathology of the lesion described above with C6709 supernatant or purified HAP were as follows: moderate number of RBC was present and diffusely scattered throughout the skin, vascular lesions were common in the affected area and RBC emigration occurred along with capillary necrosis, muscle layer was accompanied by acute myofiber degeneration and necrosis [Table/Fig-4c]. On the other hand, no such histopathological changes were found when mouse treated with TSB or Tris-HCl and showed normal skin architecture [Table/Fig-4d] suggesting further that the distinct haemorrhagic lesions observed in the mouse was most probably due to the presence of HAP.
Subcutaneous mouse assay. a) Dose dependent response with: C, buffer; 1, 125 μg; 2, 250 μg; 3, 500 ng; 4, 1 μg; 5, 2 μg; 6, 4 μg and 7, 8 μg of purified HAP were injected under the skin of each suckling mouse, respectively. b) The culture supernatants (0.1 ml) of strains grown in TSB at 37°C under shaking condition for 18 h 1) C6709 and 2) TSB medium control were injected under the skin of suckling mouse. c) Histology of mouse skin treated with culture supernatant of C6709. Thinning of epidermis with damaged keratin layer, odematous dermis with erythrocytes present throughout the dermal layer including muscle layer. d) Histology of skin treated with TSB medium showing normal skin architecture.
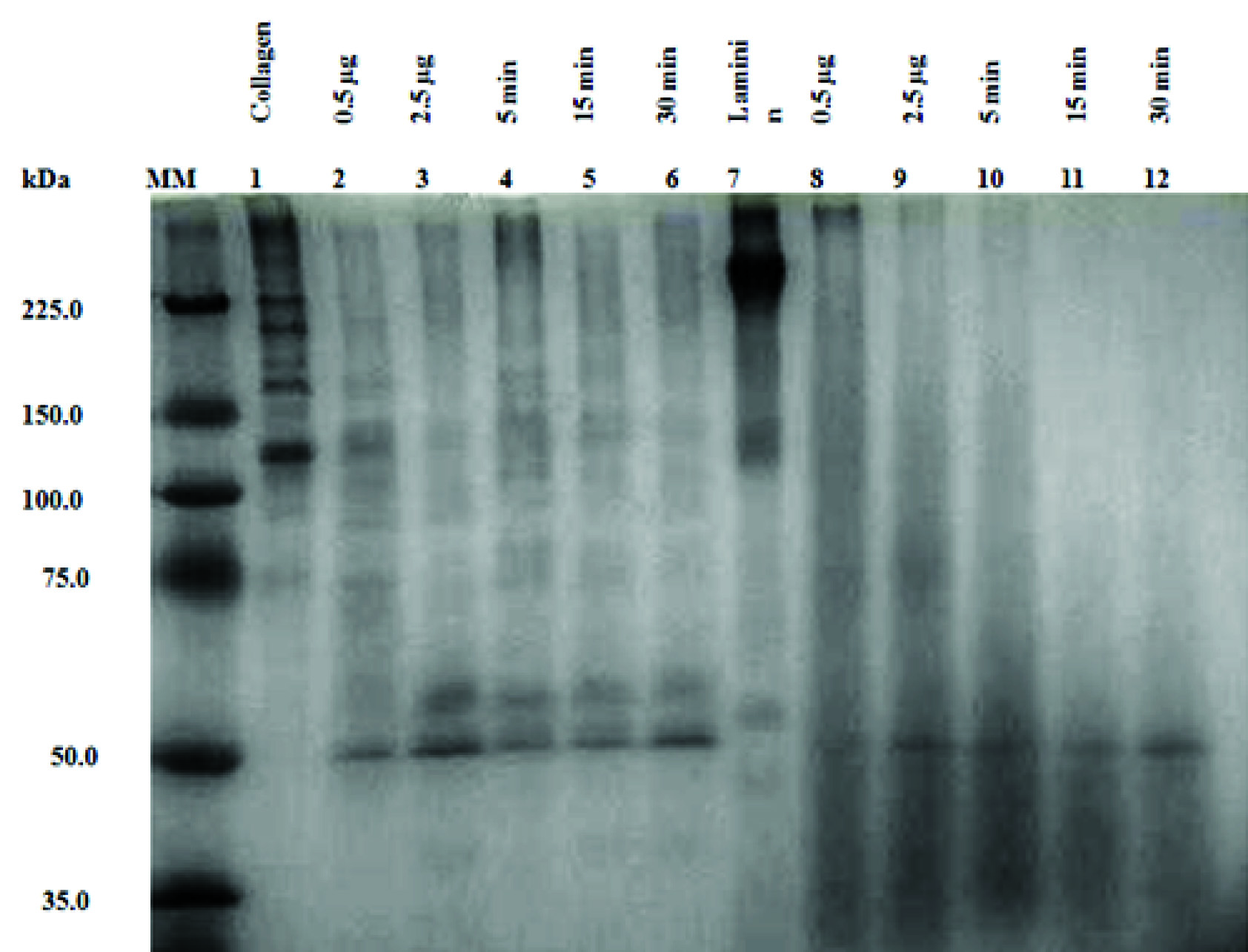
HAP degrades laminin and type IV collagen. Both the proteins were rapidly degraded by purified HAP in a dose dependent manner as shown in [Table/Fig-5]. In contrast, no such degradation of type IV collagen or laminin was observed in the absence of HAP in a reaction mixture ([Table/Fig-5] and lane 1, 7). When 2.5 μg of HAP ([Table/Fig-5] and lanes 3) (maximum concentration used in this study) was used it degraded the collagen protein at 5, 15 and 30 min at 37oC incubation temperature ([Table/Fig-5] and lanes 4, 5, 6), however, no degradation of type IV collagen occurred at 0 min ([Table/Fig-5] and lane 1). Similar results were obtained when laminin was used in the reaction mixture instead of type IV collagen, i.e., HAP degraded the laminin in a dose dependent manner and time dependent manner as shown in [Table/Fig-5] and lane 7-12).
SDS-PAGE analysis of degradation of laminin and collagen type IV by purified HAP. Purified type IV collagen (60 μg) was incubated at 37°C with purified 0.5 μg HAP (lane 2) and 2.5 μg for 0 min (lane 3), 5 min (lane 4), 15 min (lane 5) and 30 min (lane 6) and the samples were run in a 12.5% SDS-PAGE. Purified laminin (60 μg) was incubated at 37°C with purified 0.5 μg HAP (lane 8) as well as with 2.5 μg HAP for 0 min (lane 9), 5 min (lane 10), 15 min (lane11) and 30 min (lane 12). Purified type IV collagen and laminin was run as control in Lane 1 and Lane 7 respectively.
Discussion
HAP (MW 69.3kDa) is encoded by 1,827bp long hapA gene. HAP is produced by V. cholerae O1 as well as non-O1 serotype [28]. In this study we have purified the HAP from a clinical strain C6709 of V. cholerae using a simple one step anion exchange chromatography. Additionally we have taken care to remove the OMVs from the culture supernatant of the strain C6709 by ultra-filtration, which was followed by purification of HAP by using only a single step anion exchange chromatography [Table/Fig-1a]. It is to be noted that leucine aminopeptidase, a Zn-dependent metalloexopeptidase [29], the molecular size of which is close to that of HAP was retained in the DE-52 column as reported previously [16]. Thus, HAP is eluted in the non-binding fraction when loaded in a DE-52 column. HAP purified by this simple procedure was confirmed by several methods including strong protease activity as measured by azocasein assay [16], haemagglutinin assay [21] and N-terminal sequencing of the protein.
Vibrio cholera pathogenicity in extraintestinal infection like Septicemia and cellulitis is not clearly understood. Previously we have shown that the purified HAP had an inflammatory response with haemorrhage in the Rabbit Ileal Loop (RIL) assay [16]. Histopathological examination of the ileal section from the RIL assays with purified HAP showed infiltration of inflammatory cells into the gut mucosa.
In this study using a modified mouse model, we report that the HAP may be the causative agent of V. cholerae extraintestinal infection like skin lesion and haemorrhagic reaction [7]. We have used 4-5-day-old mice instead of 7-11-week-old mice because we could observe the haemorrhagic response better in older mice. We showed that when the culture supernatant of C6709 or the purified HAP from this strain was injected subcutaneously in the abdominal side of 4-5-day-old suckling mice, both of them produced distinct haemorrhagic response within 1-2h compared to placebo [Table/Fig-4a&b]. This response was due to HAP which was further confirmed when we failed to get any such effect when TSB media was injected similarly in suckling mouse abdominal skin [Table/Fig-4b]. A zinc metalloprotease (VVP) of Vibrio vulnificus enhance vascular permeability and trigger haemorrhagic response. VVP is the causative agent for skin lesion [30]. The purified HAP of C6709 or its culture supernatant in suckling mice showed thinning of epidermis with damaged keratin layer, and oedematous dermis with erythrocytes throughout the dermal layer including muscle cells [Table/Fig-4c]. HAP also caused degradation of laminin and type IV collagen, which are the major components of basement membrane of the capillary blood vessels [Table/Fig-5]. Previously it was shown by several groups that HAP can cleave several physiologically important substrates, including mucin, fibronectin, and lactoferrin [1].
In an earlier study we reported that purified 35-kDa HAP showed accumulation of haemorrhagic fluid in RIL, with histopathological studies demonstrating the presence of red blood cells, neutrophils, and eosinophils [16]. Our studies on the effect of HAP on skin tissue further confirm that HAP can cause damage to capillary blood vessels by damaging the collagen and laminin, the major components of the basement membrane of capillary blood vessels.
Conclusion
In conclusion we have determined that, V. cholerae HAP degraded the collagen and laminin of blood vessel basement membrane. As a result they can invade in host, then cause haemorrhagic reaction and extraintestinal infection. This study confirms that Vibrio cholera as a sole pathogen can cause the extra-intestinal infection. This information is important for public health notification. Besides this, result indicates appropriate testing for Vibrio cholerae and intervention are important for the patient management.