One half of all central nervous system ependymomas arise within the spinal canal [1,2]. Spinal ependymomas are common in adults, unlike the paediatric age group where ependymomas arise more often intracranially [3]. Most of the spinal ependymomas are intramedullary, of which 40% arise from the filum terminale [4].
Our understanding of these tumours has been on the basis of pioneering work of Cushing, Bailey and Kernonhan [5–7].
The myxopapillary variant of spinal ependymoma almost exclusively occurs in the lumbosacral region [8]. Papillary or myxopapillary ependymomas of the lumbosacral region are generally regarded as relatively benign slow growing tumours. In the International histological classification of tumours published by the World Health Organization this tumour variant is histologically designated as Grade I. These tumours have been diagnosed and surgically addressed for more than a hundred years.
Even though there are subtle differentiating points between spinal tumours in the conus medullaris and cauda equina regions, they usually present with a combination of symptoms [9]. The recent improvement in neuro-imaging techniques has allowed these lesions to be diagnosed early and accurately [10]. Direct surgical removal of these tumours is perhaps the most straight forward and logical approach to the problem [11]. With the advent of operating microscope, development of microsurgical techniques, imaging technologies and intraoperative neurophysiology the strategy for these neoplasms is further evolving. In the group of intramedullary tumours, spinal ependymomas are amongst those for which total surgical removal has given satisfying clinical results [4,12]. Long term control is best achieved by gross total removal at the initial operation [12].
Although it is widely believed that the desirable treatment is total excision, there is as yet no consensus on the management of incompletely excised tumour. Opinions regarding radiotherapy are controversial and the indications are empirical [13,14]. Despite the risk of local recurrence and CSF dissemination, the overall prognosis for lumbosacral intradural ependymomas appears to be very good with survival rates in the range of 95% over 10 years [15]. In the present study, we investigate the clinical characteristics and long-term outcomes in patients with conus cauda ependymoma that were managed at our center with baseline comparison of our findings with those reported in literature.
Materials and Methods
Study design and cohort: A retrospective, single-institutional study conducted at BYL Nair Hospital, Mumbai involving 44 patients undergoing surgical excision for conus cauda ependymoma between January 2001 and December 2015 was performed. Ethical approval was obtained from the institutional ethical board committee prior to initiation of the study. All consecutive cases of histologically confirmed ependymoma cases in the conus medullaris and cauda equina region were included in our study.
Data-extraction: This included review of patient medical records for eligible patients. Detailed scrutiny and analysis of the patient’s data with respect to the demographic features, clinical findings, and investigative procedures, extent of surgical resection, intra and postoperative complications, efficacy of adjuvant therapy, postoperative results and long term follow-up were done [Table/Fig-1].
Patient characteristics of 44 patients with conus cauda ependymoma seen at our institute between 2001- 2015.
| Sr. No. | Characteristic | Value |
|---|
| 1. | Age, in years Mean ± SD Range | 30.95 ± 12.78Max 63, Min 3 |
| 2. | Gender, n(%) Male Female | 35 (80%)9 (20%) |
| 3. | Symptom duration, in months Mean ± SD Range | 9.52± 9.27Max 36, Min 1 |
| 4. | Presenting Symptoms, n(%) Back pain Lower limb weakness Difficulty in walking Sensory symptoms Bowel/bladder complaints Sexual dysfunction Deformity | 40 (91%)37 (84%)36 (82%)19 (43%)15 (34%)6 (14%)3 (7%) |
| 5. | Presenting Signs, n(%) Motor weakness in lower limbs Altered tone Hypotonia Hypertonia Deranged Reflexes Absent Brisk Lower limb hypoesthesia | 37 (84%)24(55%)4(9%)27(61%)3(7%)24(55%) |
| 6. | Surgical Resection, n (%) Total Near Total Subtotal | 39 (88.6%)04 (9.1%)01 (2.3%) |
| 7. | Length of stay, days Mean ± SD Range | 12.07± 14.01Max 85, Min 3 |
| 8. | Postoperative complications, n(%) Wound infection Urinary retention Motor weakness Dysesthesia | 2 (4.5%)3 (7%)2 (4.5%)1 (2.3%) |
| 9. | Histopathology, n(%) Myxopapillary Ependymoma Cellular Ependymoma Clear Cell Ependymoma Anaplastic Ependymoma | 24 (55%)17 (39%)1 (2.3%)2 (4.5%) |
| 10. | Follow up, in months Mean± SD Range | 22.23± 11.32Max 48 Min 6 |
| 11. | Recurrence Rate, n(%) | 2 (4.5%) |
| 12. | Long term functional outcome, n(%) Good Fair Poor | 28(63.6%)9(20%)7(15%) |
Study Protocol: In all patients who underwent surgery, routine hematological workup and Magnetic Resonance Imaging (MRI) scan of the lumbosacral spine was performed. Laminectomies with respect to the extent of tumour were done in all patients in our series. After opening the dura in midline, the edges were reflected away and held with stay sutures. The excision of tumour was done in a piecemeal fashion, preserving the nerve rootlets. Based on the involvement/ infiltration of nerve rootlets and conus by the tumour, the extent of excision was subtotal to total. Once the excision was complete, the dura was closed in a water tight manner with absorbable vicryl sutures. Postoperative neurological status and complications were studied. In our series MRI scan was done in all 44 patients during their follow up visits. Preoperative and postoperative neurological and functional outcome were studied and compared.
Follow-up was obtained either by clinical records or by personal communication through letters. Patients with subtotal tumour excision were subjected to adjuvant radiotherapy. Radiotherapy was deferred in those patients where complete tumour excision was performed and these patients were closely observed. All such patients were monitored with serial MRI scans to detect any recurrence during subsequent follow-up visits. In our series four patients had no follow-up after surgery and were hence excluded from our study.
Statistical Analysis
Descriptive statistics were employed for projection of results in the form of frequencies and proportions of categorical variables and mean± SD (range) for quantitative variables.
Results
Back pain and lower limb weakness were the predominant presenting symptoms. Sphincter disturbances in the form of incontinence/retention of urine, enuresis and constipation were present in 15 (34%) patients. Sexual dysfunction in the form of impotency and premature ejaculation was noted in 6 patients. In 2 patients, there was kyphotic deformity of the lumbar spine and 1 patient had valgus deformity of the foot at the time of presentation to us [Table/Fig-1].
In 39 patients, the excision of tumour was complete (total). In 1 patient subtotal excision was performed as the tumour was infiltrating the rootlets and conus medullaris. In 4 patients, the excision was near total and a small portion of the tumour was left behind, that was densely adherent to the rootlets [Table/Fig-1,2].
Intraoperative finding in our series.
| Sr.No. | Age(Years) | Sex(M/F) | Operative Procedure | Histopathology Report |
|---|
| Procedure | Findings | Type of Resection |
|---|
| 1 | 25 | M | D12-L3 laminectomy | Soft, Fleshy, vascular tumour | Total | Myxopapillary ependy |
| 2 | 45 | M | D12-L1 laminectomy | Soft, Fleshy, vascular tumour | Total | Cellular ependymoma |
| 3 | 40 | F | L4-S1 laminectomy | Firm and mildly vascular | Total | Myxopapillary ependy |
| 4 | 38 | M | D12-L3 laminectomy | Soft, Fleshy, vascular tumour | Near total | Cellular ependymoma |
| 5 | 30 | M | D12-L2 laminectomy | Soft, Fleshy, vascular tumour | Near total | Myxopapillary ependy |
| 6 | 29 | M | L3-S3 laminectomy | Soft, Fleshy, vascular tumour | Total | Myxopapillary ependy |
| 7 | 38 | M | D12-L3 laminectomy | Firm, Fleshy, vascular tumour | Total | Myxopapillary ependy |
| 8 | 40 | F | L1-L2 laminectomy | Soft, Fleshy, vascular tumour | Total | Cellular ependymoma |
| 9 | 32 | M | L1-L2 laminectomy | Soft, Fleshy, vascular tumour | Total | Myxopapillary ependy |
| 10 | 50 | M | L3-S1 laminectomy | Soft, Fleshy, vascular tumour | Total | Myxopapillary ependy |
| 11 | 40 | M | L3-S1 laminectomy | Partly solid and partly cystic | Near total | Anaplastic ependymoma |
| 12 | 15 | M | L1-S2 laminectomy | Firm and mildly vascular | Total | Myxopapillary ependy |
| 13 | 51 | M | L1-L3 laminectomy | Firm and mildly vascular | Total | Myxopapillary ependy |
| 14 | 27 | F | D12-L5 laminectomy | Soft, Fleshy, vascular tumour | Total | Myxopapillary ependy |
| 15 | 3 | M | L1-L5 laminectomy | Firm and mildly vascular | Subtotal | Anaplastic ependymoma |
| 16 | 63 | M | L3-S1 laminectomy | Soft, Fleshy, vascular tumour | Total | Cellular ependymoma |
| 17 | 35 | M | L2-L4 laminectomy | Soft, Fleshy, vascular tumour | Total | Myxopapillary ependy |
| 18 | 35 | M | L1-L4 laminectomy | Soft, Fleshy, vascular tumour | Total | Cellular ependymoma |
| 19 | 20 | M | D12- L3 laminectomy | Soft, Fleshy, vascular tumour | Total | Myxopapillary ependy |
| 20 | 36 | M | L2-L5 laminectomy | Soft, Fleshy, vascular tumour | Total | Myxopapillary ependy |
| 21 | 14 | M | L1-L3 laminectomy | Soft, Fleshy, vascular tumour | Total | Myxopapillary ependy |
| 22 | 22 | M | L1-L4 laminectomy | Soft, Fleshy, vascular tumour | Total | Cellular ependymoma |
| 23 | 26 | M | L1-L3 laminectomy | Soft, Fleshy, vascular tumour | Total | Myxopapillary ependy |
| 24 | 41 | M | L1-L2 laminectomy | Soft, Fleshy, vascular tumour | Total | Cellular ependymoma |
| 25 | 12 | M | L1-S3 laminectomy | Soft, Fleshy, vascular tumour | Total | Myxopapillary ependy |
| 26 | 27 | M | D12-L1 laminectomy | Soft, Fleshy, vascular tumour | Total | Myxopapillary ependy |
| 27 | 15 | M | L1-L4 laminectomy | Soft, Fleshy, vascular tumour | Total | Myxopapillary ependy |
| 28 | 50 | F | D12-L2 laminectomy | Soft, Fleshy, vascular tumour | Total | Cellular ependymoma |
| 29 | 45 | M | L1-L3 laminectomy | Soft, Fleshy, vascular tumour | Total | Cellular ependymoma |
| 30 | 28 | M | L1-L3 laminectomy | Soft, Fleshy, vascular tumour | Total | Cellular ependymoma |
| 31 | 35 | F | L1-L3 laminectomy | Soft, Fleshy, vascular tumour | Total | Cellular ependymoma |
| 32 | 45 | M | D12-L2 laminectomy | Soft, Fleshy, vascular tumour | Total | Myxopapillary ependy |
| 33 | 17 | M | L1-L3 laminectomy | Soft, Fleshy, vascular tumour | Total | Cellular ependymoma |
| 34 | 34 | M | D12-L2 laminectomy | Firm, Fleshy, vascular tumour | Near total | Myxopapillary ependy |
| 35 | 25 | F | D12-L2 laminectomy | Firm, Fleshy, vascular tumour | Total | Cellular ependymoma |
| 36 | 50 | F | L1-L2 laminectomy | Firm, Fleshy, vascular tumour | Total | Cellular ependymoma |
| 37 | 27 | M | D12-L2 laminectomy | Firm, Fleshy, vascular tumour | Total | Cellular ependymoma |
| 38 | 34 | M | D12-L2 laminectomy | Firm, Fleshy, vascular tumour | Total | Myxopapillary ependy |
| 39 | 24 | M | L1-L2 laminectomy | Firm, Fleshy, vascular tumour | Total | Cellular ependymoma |
| 40 | 19 | M | L4-S1 laminectomy | Firm, Fleshy, vascular tumour | Total | Myxopapillary ependy |
| 41 | 22 | M | D12-S1 laminectomy | Firm, Fleshy, vascular tumour | Total | Myxopapillary ependy |
| 42 | 31 | F | L1-L5 laminectomy | Firm, Fleshy, vascular tumour | Total | Clear cell ependymoma |
| 43 | 13 | F | D12-L5 laminectomy | Firm, Fleshy, vascular tumour | Total | Cellular ependymoma |
| 44 | 14 | M | D12-L2 laminectomy | Firm, Fleshy, vascular tumour | Total | Myxopapillary ependy |
In majority of patients at follow-up, back pain and motor weakness improved, when compared with their preoperative status. However, the sphincter problem improved in only 25% patients after the surgery [Table/Fig-3].
The symptom wise progression during follow up in our series population.
| Symptom | Improvement | No improvement | Worsening |
|---|
| Pain (N= 40) | 36 (90%) | 4 (10%) | 0 |
| Motor weakness (N= 37) | 26 (70%) | 7 (19%) | 4 (11%) |
| Sensory disturbances (N= 19) | 10 (53%) | 8 (42%) | 1 (5%) |
| Bowel/bladder comp (N=15) | 3 (20%) | 11 (73%) | 1 (7%) |
During follow-up visits in 39 (89%) patients there was no evidence of residual or recurrent tumour on MRI scans. In these patients, total excision of the tumour was done. These patients were clinically asymptomatic. In 5 (11%) patients there was residual tumour on follow-up MRI scans. The extent of excision in these patients was near total to subtotal. Clinically they were pain free, but the motor weakness in lower limbs and sphincter disturbances persisted. These patients were subjected to Radiotherapy (RT). In 2 patients, recurrence was noted at 1 year follow-up. Both these patients were histologically of anaplastic variant. They were symptomatic and motor weakness in lower limbs had deteriorated. One patient of which was re-operated and near total excision of the tumour was performed. MRI scan of second patient revealed a large recurrence of tumour and was advised surgery. He however failed to follow-up with us.
In assessing long-term results the Beniamino Classification was used [16].
Good- (70-90% of normal): Minimal neurological abnormality such as weakness of limbs, sensory abnormality, mild sphincter trouble, walks unaided witho ut support and is independent.
Fair- (50-70% of normal): Moderate neurological deficit walks with stick or crutches, reasonably independent.
Poor- (30-50% of normal): Gross neurological deficits, walks with support, has gross sphincter and motor disturbances, totally dependent on others for life.
Most of the patients who had poor functional outcome were having severe neurological deficits at the time of presentation [Table/Fig-1]. This was despite total excision of the tumour. Patients who had fair functional outcome were predominantly those who had residual or recurrent tumours. Majority of these patients had minimal neurological deficits at the time of presentation and excision of the tumour was near total to total.
Illustrative Cases
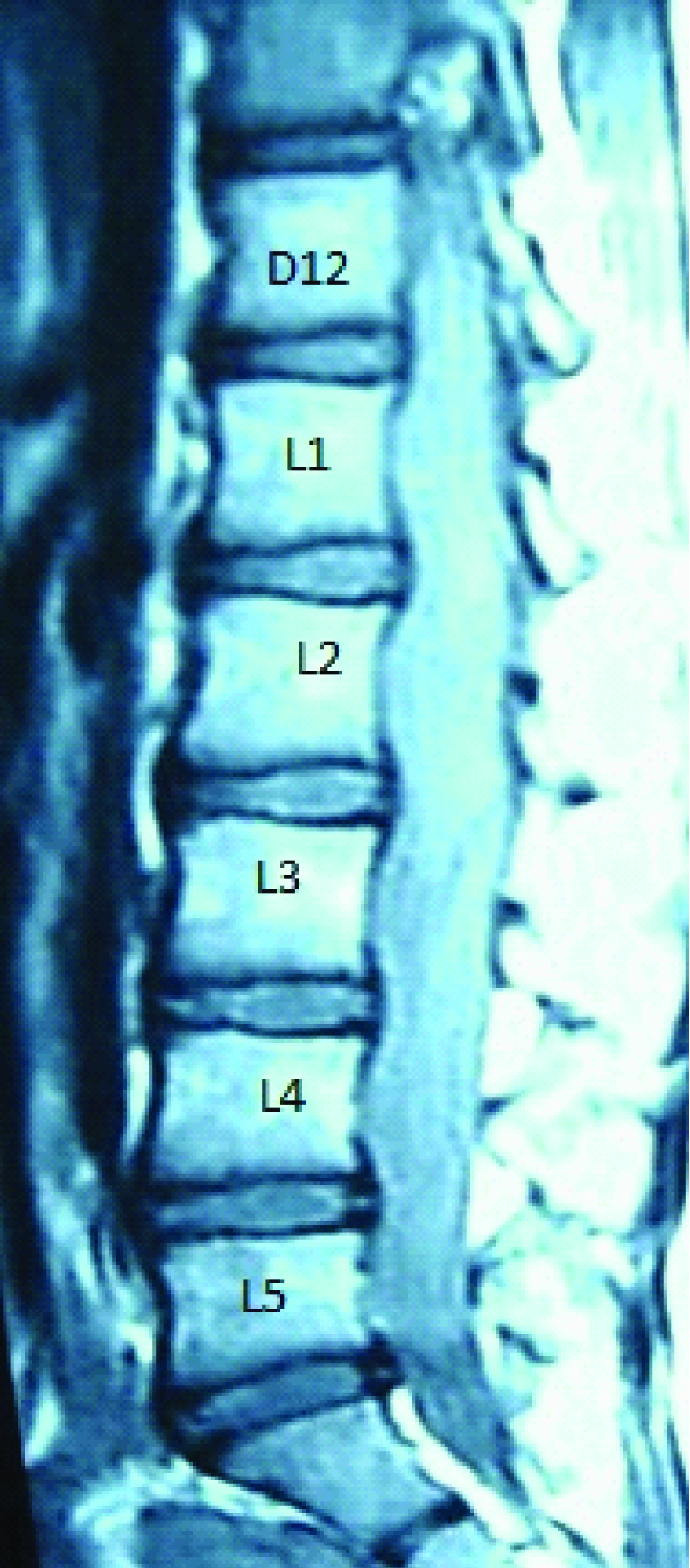
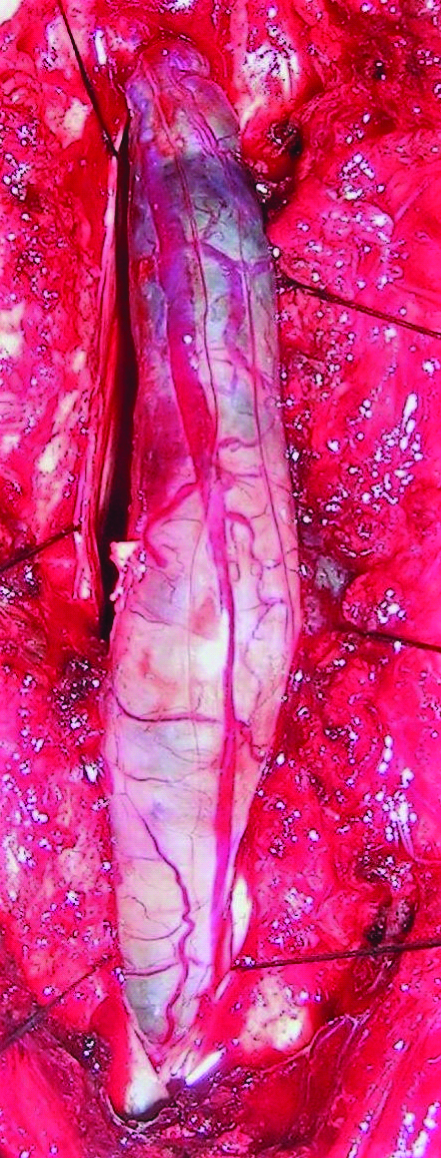
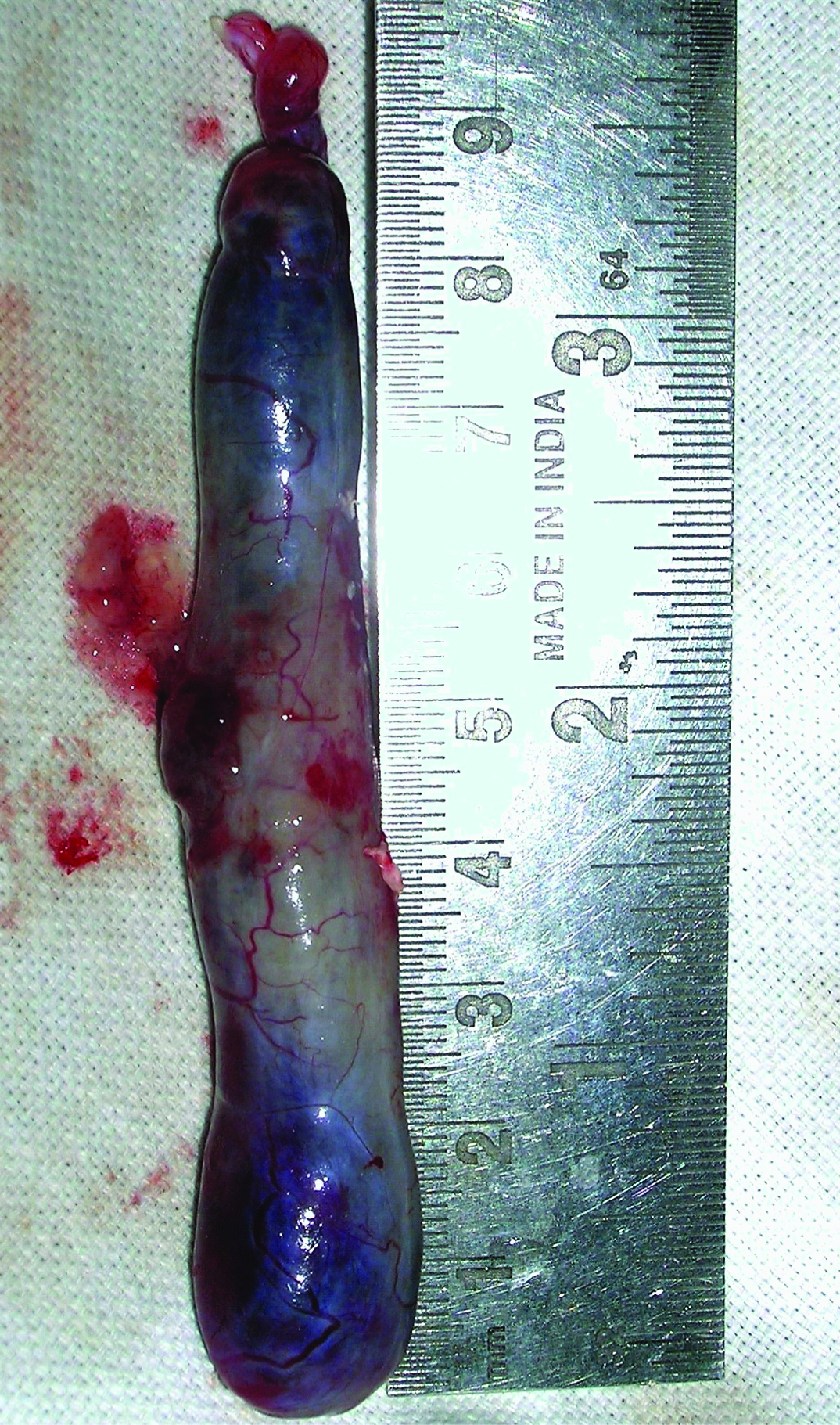
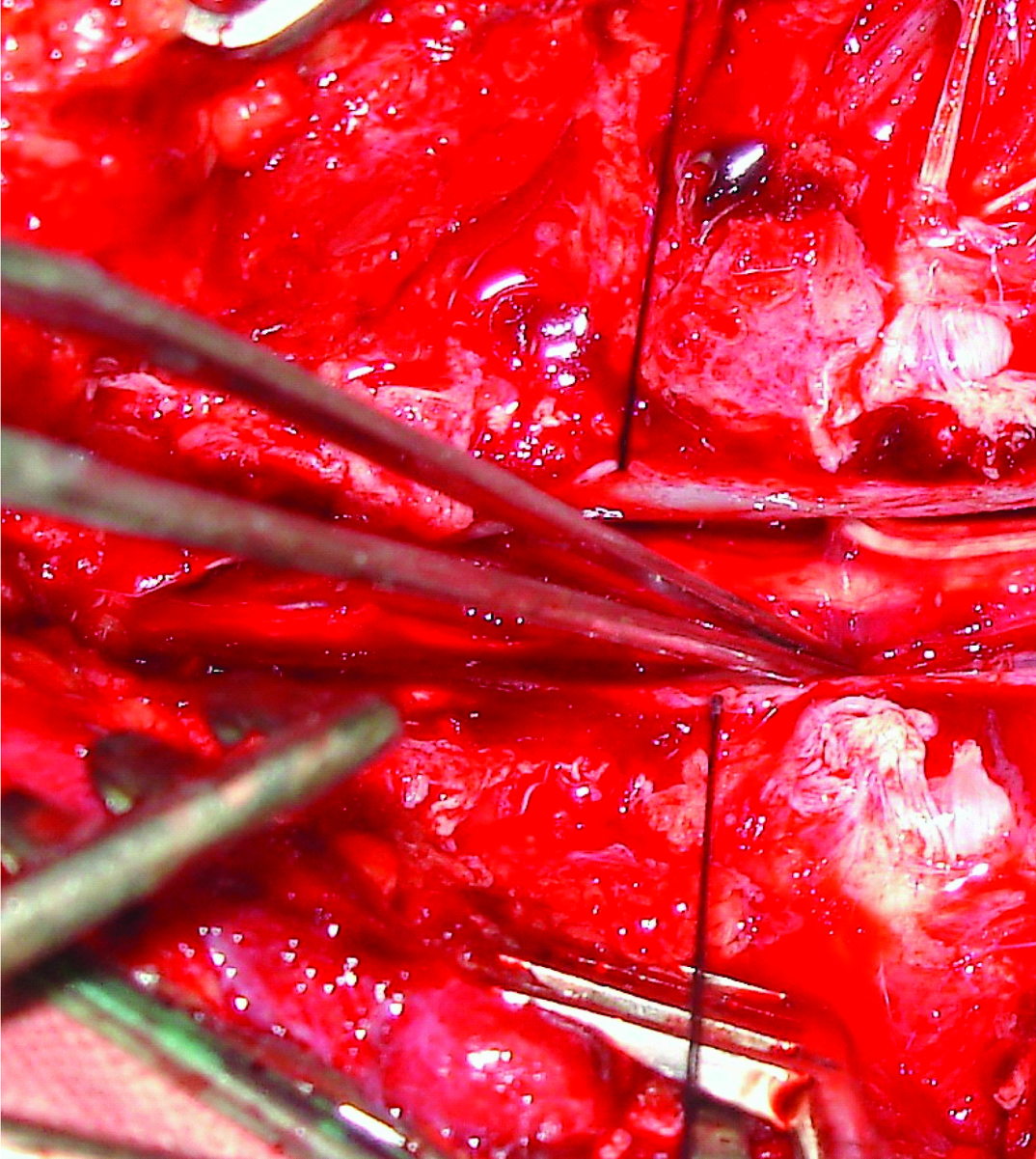
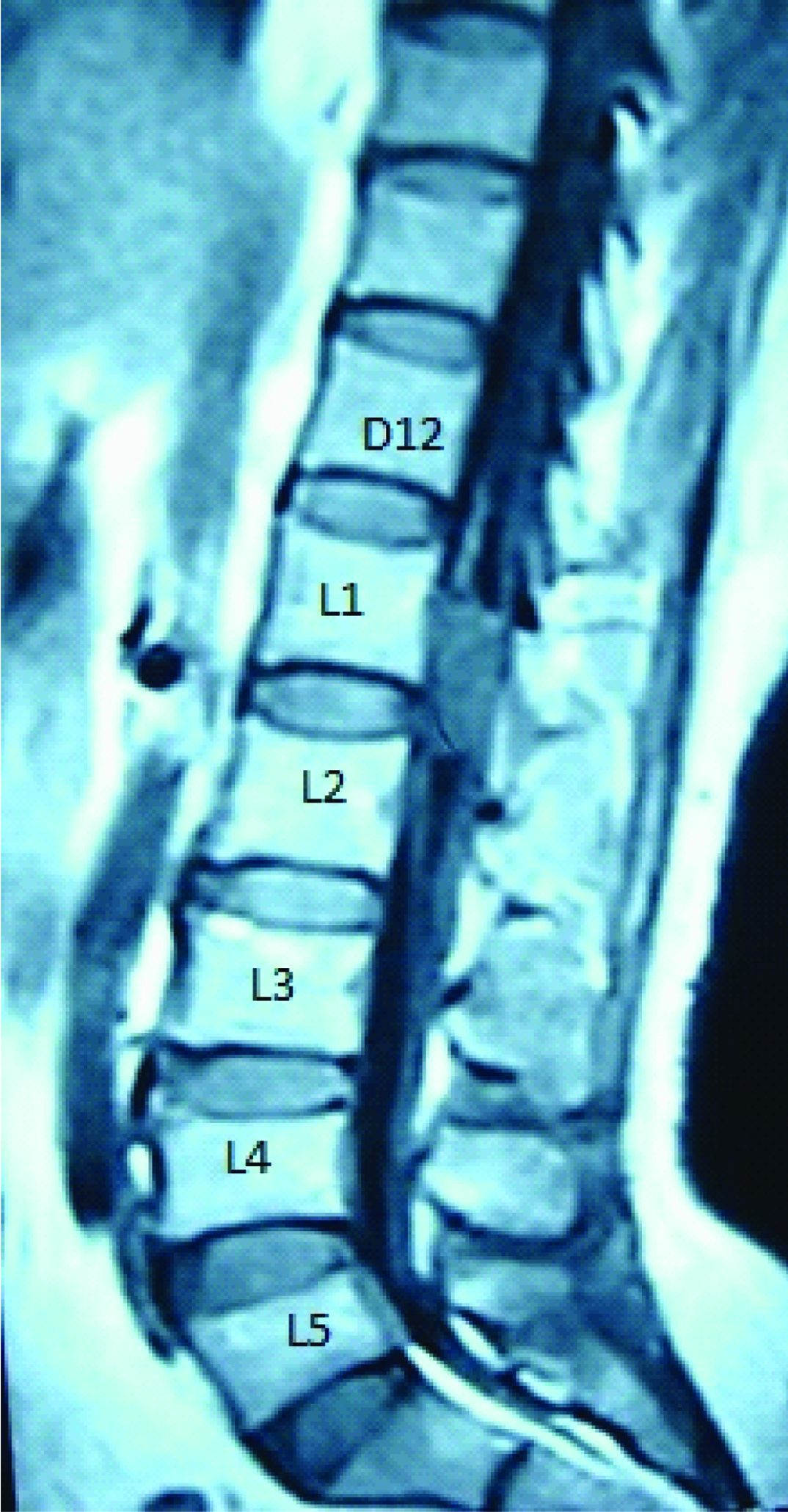
Case 1: A 27-year-old female presented to us with back pain and difficulty in walking. MRI showed an homogenously enhancing SOL from D12- L5 [Table/Fig-4]. D12- L5 laminectomy with midline durotomy was done. The tumour was visualized [Table/Fig-5] and total excision of tumour was done. The tumour was soft, fleshy and vascular. [Table/Fig-6]. Following tumour excision the rootlets were decompressed [Table/Fig-7]. The postoperative MRI showed complete tumour excision [Table/Fig-8]. Patient improved in her symptoms.
Shows post contrast T1W sagittal image of the lumbosacral region with the ependymoma at D12-L5 level. The lesion enhances homogenously following contrast administration.
Shows tumour seen protruding out on opening the dura. The ependymoma is fleshy and reddish purple in colour.
Gross specimen showing the dimensions and character of the tumour.
Shows nerve rootlets and the conus medullaris on total excision of the ependymoma.
Shows postoperative sagittal T1 image demonstrating complete excision of the tumour with no evidence of any residue.
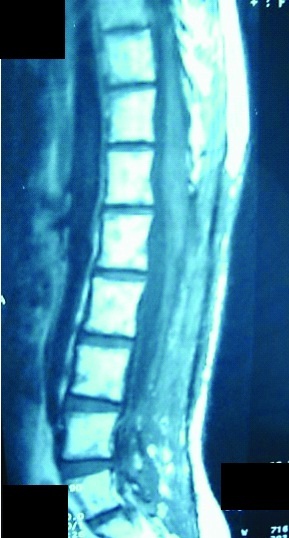
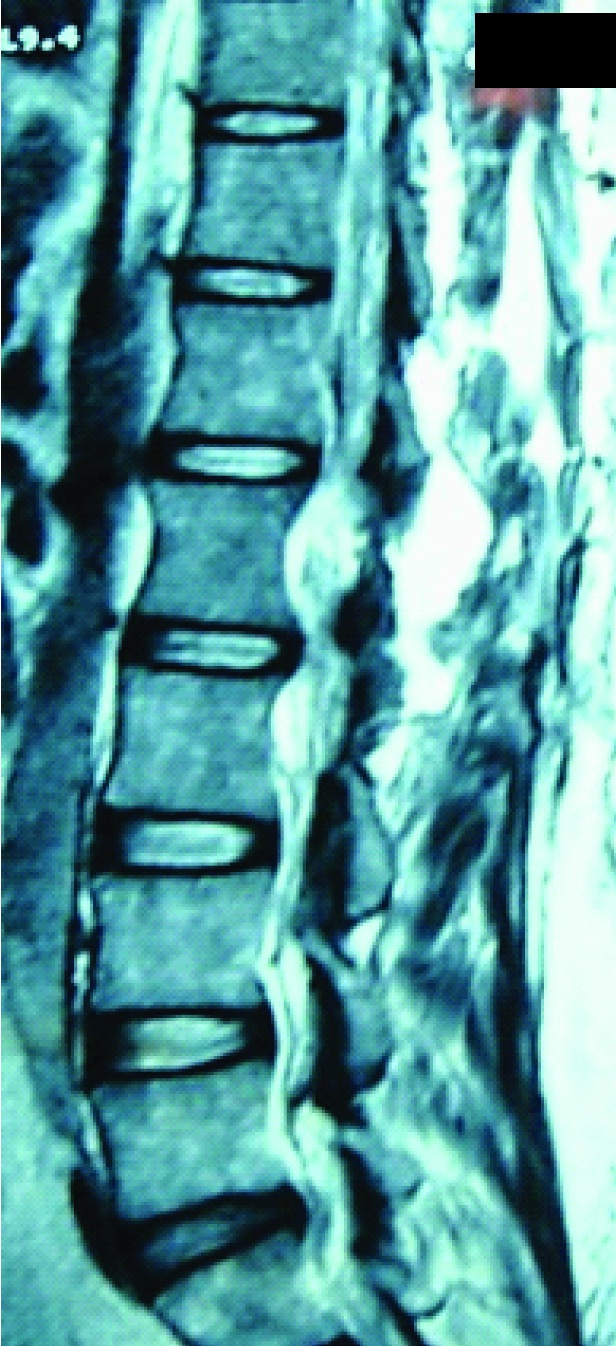
Case 2: A 45-year-old male with back pain, difficulty in walking and frequency of micturition had a short segment tumour at L1-L2 level [Table/Fig-9]. Laminectomy with total excision of tumour was performed as seen in Postoperative MRI [Table/Fig-10]. Patient had a good outcome with complete relief in symptoms.
Shows post contrast T1W sagittal image of the lumbosacral region with the ependymoma at L1-2 level. The lesion enhances homogenously following contrast administration.
Shows postoperative T2W sagittal image with total excision of tumour and CSF filled cavity.
Discussion
Spinal cord tumours have a long and well documented history. Victor Horsley is credited with the first successful removal of a spinal cord tumour in 1887 [5]. Cushing was the first to remove an intramedullary ependymoma in 1905 [5]. Bailey was the first to describe ependymoma in 1924. Elsberg stressed that tumours with well defined planes allow for total removal [17]. Kernohan in 1932 was probably the first to use the term myxopapillary for those ependymomas of the lumbosacral region, where mucinous change was conspicuous [7].
We have retrospectively analysed our series of 44 cases of conus cauda ependymoma and have compared our results with 3 other series in world literature: Mathew and Todd, Sonneland et al., and Mork et al., [4,11,18] [Table/Fig-11].
Patient characteristics from relevant literature depicting outcomes in patients with conus cauda ependymoma.
| Authors,year | SampleSize (n) | Studyduration | Age Range(median), in years | FemaleGendern (%) | Symptomduration range(median), months | Presentingsymptoms(%) | Surgicalresection n (%)/ RT (%) | Recurrencerate (%) |
|---|
| Mathew & Todd, 1993 [18] | 7 | 1971- 1989 | 9 – 52(37) | 1 (14.3) | 1-36 (3) | BP (71)SMD (86)BBD (71) | GTR – 1(14)/0STR – 6(86)/83 | Data unavailable |
| Sonnneland et al., 1985 [11] | 77 | 1924- 1983 | 6 – 82(36.4) | 29 (37.7) | 1-360 (18) | BP (96)SMD (53)BBD (3)SD (36) | GTR – 45(58)/82STR – 32(42)/84 | 9 |
| Mork et al., 1977 [4] | 32 | 1953- 1974 | 3 – 80(32) | 12 (37.5) | 1 – 120 (12) | BP -Commonest | GTR – 32(54)/85STR – 11(46)/90 | Data unavailable |
| Present study | 44 | 2001 - 2015 | 3 – 63(31) | 9 (20.5) | 1 – 36 (10) | BP (91)SMD (84)BBD (34)SD (14) | GTR – 39(89)/0STR – 5(11)/100 | 5 |
BP- Back Pain, SMD- Sensory Motor disturbances, BBD – Bowel bladder disturbances, SD- Sexual disturbances, GTR- Gross total Resection, STR – Subtotal resection
The presentation of symptoms in our series was early when compared with Sonneland and Mork et al., as we had the advantage of having MRI scan at our discretion [4,11]. Hence, the diagnosis of tumour was relatively early.
In our series total excision of the tumour was done in 39 (89%) patients. In only 5 patients near total to subtotal excision was done (11%). This was possible due to routine use of microscope and microsurgical techniques. In comparison total excision was done in 45 (58%) patients by Sonneland et al., and in 32 (42%) patients subtotal to near total excision was performed [11]. Similarly; Mathew et al., and Mork et al., had total excision in 1 (14%) and 13 (54%) patients respectively [4,18]. This can be attributed to pre-microsurgical era when the use of microscope and microsurgical techniques were not very popular.
Myxopapillary variant of ependymoma was the commonest histological findings in Sonneland et al., (100%) and Mork et al., series [4,11]. In our series the incidence of myxopapillary variant was 55% followed by cellular type (39%).
In our series, radiotherapy was not given in patients where total tumour excision was performed. Radiotherapy was given in only 5 patients where the excision was near total to subtotal. Similarly, in series by Mathew et al., radiotherapy was given to patients with subtotal excision [18]. Radiotherapy was given in group with total tumour excision in addition to subtotal group in Sonneland et al., and Mork et al., series [4,11]. This was attributed by them to their philosophy of high incidence of recurrence in patients with total tumour excision who did not receive radiotherapy. Sonneland also stressed en-bloc complete excision of the tumour instead of piecemeal excision and advocated postoperative radiotherapy in cases with total excision done by piecemeal fashion.
Residual/Recurrence rates: There were no significant series except Sonneland et al., available in literature to compare the data for recurrence/ residual tumour. Sonneland found recurrence in 7 patients in their series. In 2 patients of which the excision was total, whilst in 5 patients the excision was subtotal. Radiotherapy was subsequently given in 6 patients with recurrence of tumour [11]. In our series, recurrence was noted in 2 patients in whom the excision was near total and had received radiotherapy.
Limitation
This is a retrospective study and single institute experience. These drawbacks may limit generalization of results.
Conclusion
Conus cauda ependymomas are relatively benign tumours. The long term prognosis is excellent with respect to recurrence and functional outcome in cases with complete tumour excision. Early diagnosis and surgery will prevent occurrence of permanent neurological deficits. Radiotherapy can be given in cases of subtotal excision but there is limited role of radiotherapy in cases with total tumour excision.
BP- Back Pain, SMD- Sensory Motor disturbances, BBD – Bowel bladder disturbances, SD- Sexual disturbances, GTR- Gross total Resection, STR – Subtotal resection