Ovarian cancer is the seventh most common cancer in women worldwide (18 most common cancer overall), with 239,000 new cases diagnosed in 2012 [1]. Among women in the United States, ovarian cancer is the eighth most common cancer and the fifth leading cause of cancer death, after lung and bronchus, breast, colorectal and pancreatic cancers [2]. Ovarian cancer has highest mortality rate among all gynaecological malignancy due to late diagnosis. Due to lack of early clinical symptoms around 60%-70% of women have advance disease (stage III or IV) at the time of diagnosis [3]. Parker WH and Berek J reported that postmenopausal women have 8-45% risk of an ovarian tumour being malignant compare to premenopausal women who have 7-13% such risk [4]. Funt SA et al., showed in their study that ultrasound is the primary imaging modality for the ovaries, with accuracy of up to 94% for diagnosis of malignancy [5]. Early and accurate differential diagnosis of adnexal masses, including their benign or malignant nature is important to decide early intervention among ovarian mass women. The introduction of transvaginal colour Doppler imaging with pulsed Doppler spectral analysis represented a quantum leap in technical development [6]. The aim of present study was to evaluate diagnostic accuracy of B- mode USG and Doppler scan for ovarian lesions.
Materials and Methods
The study was conducted in Department of Radiodiagnosis at tertiary teaching medical college center from March 2009 to September 2010. Ethical approval for this prospective study was obtained from local ethical committee. The patients included in the study were those referred with either palpable adnexal mass or incidentally detected adnexal masses, irrespective of age or menstrual status of the woman. Total 250 women were screened with ultrasonography (USG) and fine needle aspiration cytology, out of these 78 women underwent surgery. Only those patients who had true ovarian mass intraoperatively and on histopathology were included in study, rest masses were excluded. Patients with ectopic pregnancy were also excluded from the study.
Total 50 patients were included in the study. These patients were examined with a real time sonography equipment- Colour Doppler USG SSA 350A/31 and or Aloka prosound SSD- 4000SV, using sector transducer of frequency 3-5 MHz through a transvesical approach, B-Mode USG, Colour Doppler and spectral Doppler was performed. Morphological indexing of adnexal masses was done according to Kujrak et al., morphological scoring system [Table/Fig-1] [7]. On B- Mode USG both solid and cystic components are seen in complex adnexal masses [Table/Fig-2]. Parameters taken in Colour Doppler were flow study, vessel arrangement, and vessel morphology and vessel location [Table/Fig-3]. Parameters considered for malignancy in spectral Doppler were Resistive Index (RI) and Pulsatility Index (PI). If required, endo-vaginal sonography was also performed with a 4-8 MHz vaginal transducer. Statistical analysis was performed with SPSS version 19. Fisher’s-exact test and Chi-square with Yates correction were used.
Kujrak morphologic scoring system.
| Criteria | Sub-criteria | Unilocular | Multilocular | Complex cystic |
|---|
| Fluid | Clear | 0 | 1 | 1 |
| Internal echoes | 1 | 1 | 2 |
| Inner Margin | Smooth | 0 | 1 | 1 |
| Irregular | 2 | 2 | 2 |
| Papillary growth | Suspicious | 1 | 1 | 1 |
| Definitive | 2 | 2 | 2 |
| Solid area | Homogenous | 0 | 0 | 1 |
| Echogenic | 0 | 0 | 2 |
| Peritoneal fluid | Not present | 0 | 0 | 0 |
| Present | 1 | 1 | 1 |
| Laterality | Unilateral | 0 | 0 | 0 |
| Bilateral | 1 | 1 | 1 |
| Morphologic score | ≤2 | 3-4 | >4 |
| Benign | Equivocal | Suspicious |
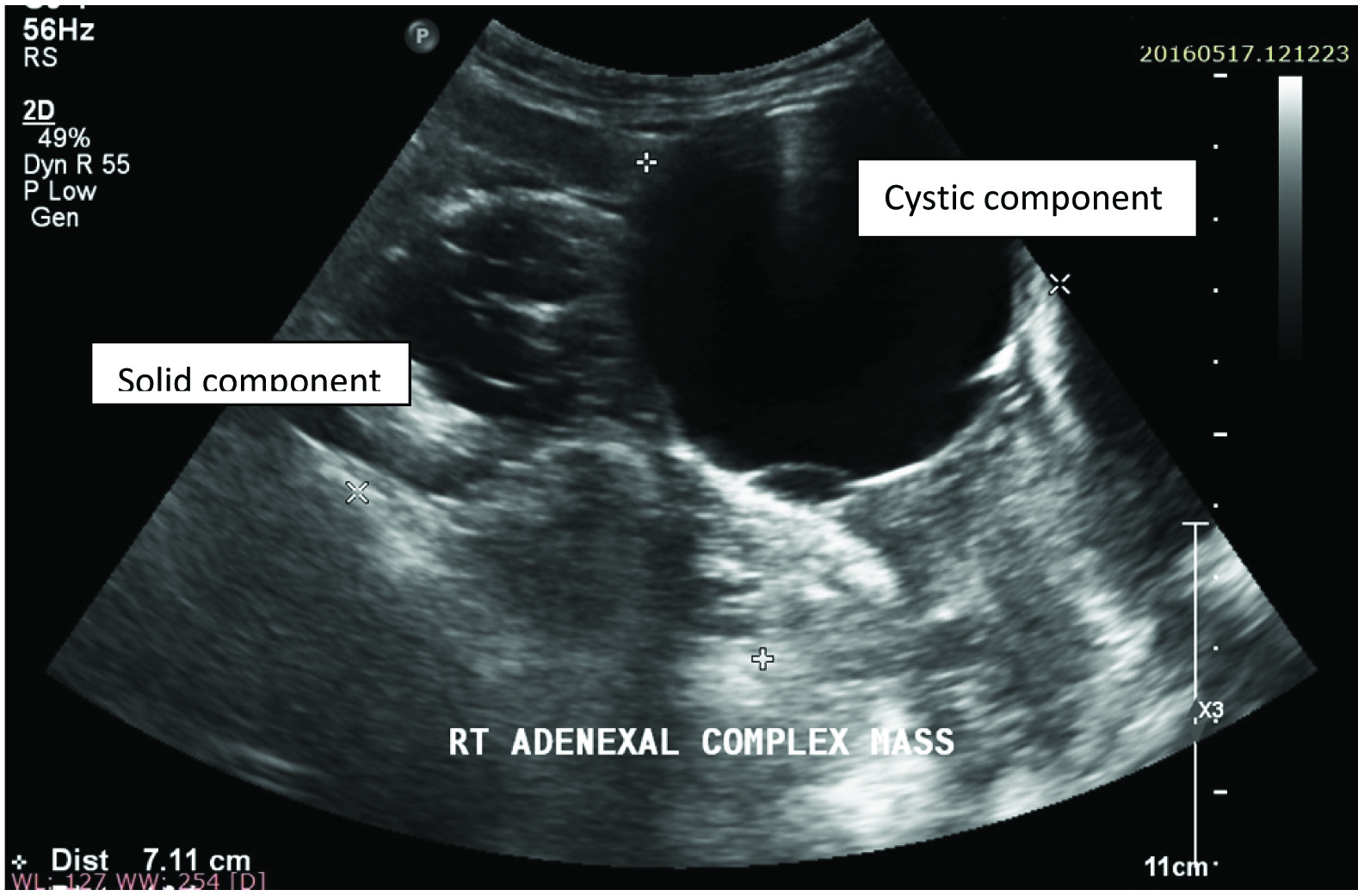
Right complex adnexal mass on B- Mode USG showing both cystic and solid components.
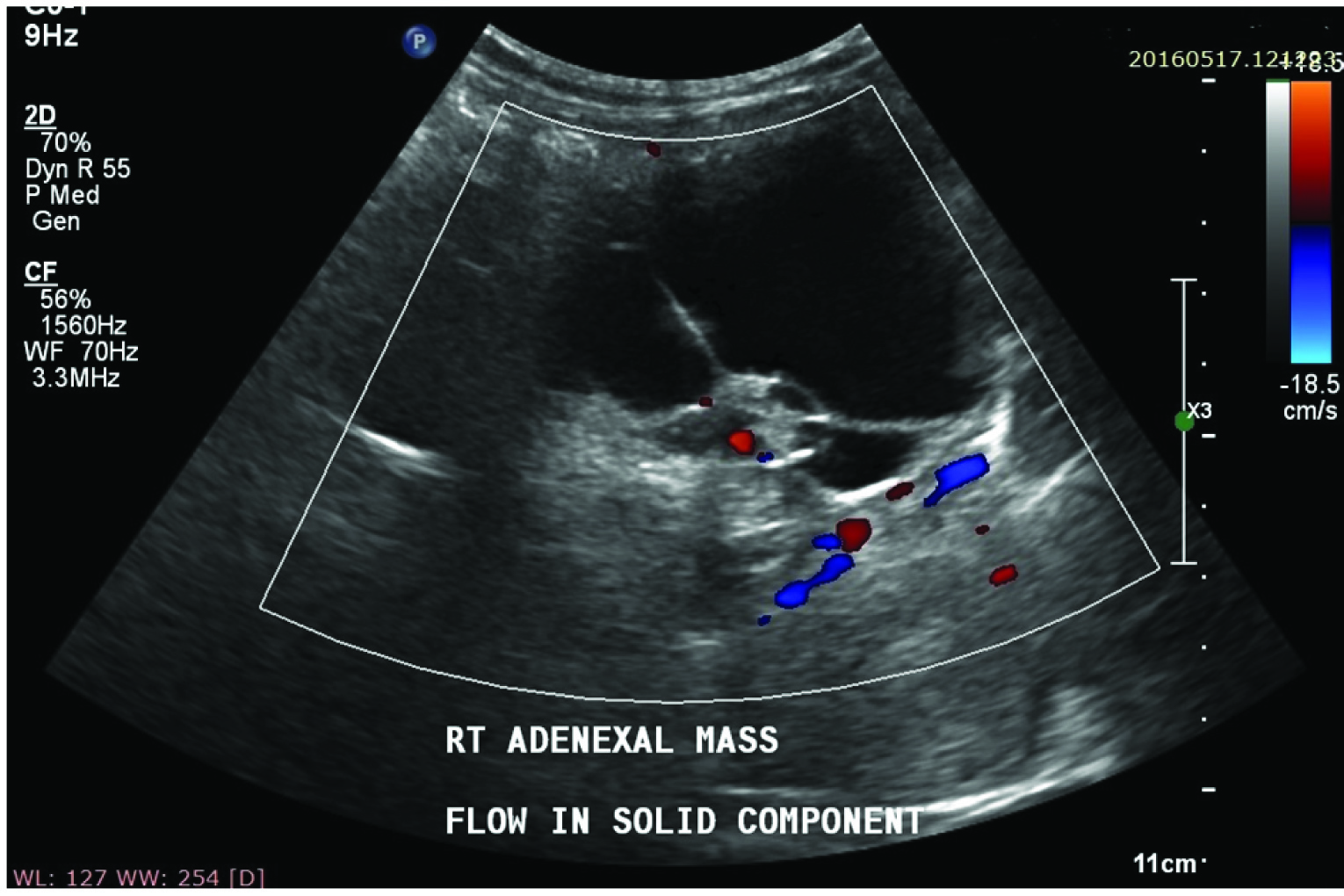
Complex adnexal mass showing flow in solid component on Colour Doppler scan.
Results
Total 50 women, from 10 years to 80 years age, were included in present study. Out of 50 women, 7 have bilateral ovarian masses with both side mass having similar histopathology. Total 30 patients have benign pathology while 20 were having malignant lesions. Out of these, 23 (46%) were premenopausal while 27 (54%) were postmenopausal. Among premenopausal women, only 2 (8.7%) were proved malignant on histopathology. Among postmenopausal women, 18 (66.7%) had malignant ovarian masses [Table/Fig-4]. Most common benign ovarian lesions were inflammatory (30%) followed by serous cystadenoma (27%) while most common malignant ovarian masses were serous carcinoma (50%) followed by mucinous carcinoma which account for 25% of malignant ovarian masses [Table/Fig-5].
Patients grouped according to menstruation status and histopathological diagnosis.
| Patients | Non Malignant | Malignant |
|---|
| Pre-Menopausal | 21 | 2 |
| Post-Menopausal | 9 | 18 |
| Total | 30 | 20 |
Statistical Analysis:- Chi-square with Yates correction = 15.06, p-value = .0001(<.05)
Histopathological nature of benign and malignant ovarian masses.
| Benign masses | N = 30 | Malignant masses | N = 20 |
|---|
| Inflammatory lesion | 9 | Serous carcinoma | 10 |
| Serous cystadenoma | 8 | Mucinous carcinoma | 5 |
| Endomatrioma | 4 | Clear cell carcinoma | 3 |
| Cystic teratoma | 4 | Dysgerminoma | 2 |
| Mucinous cystadenoma | 2 | Most common malignant – serous carcinomaMost common benign – inflammatory lesion |
| Hemorrhagic cyst | 2 |
| Functional cyst | 1 |
B-Mode USG was done in all 50 women and they were given morphological score accordingly. On B- Mode USG 10 (20%) each were unilocular and multilocular cystic, 29 (58%) were complex cystic and one (2%) was solid ovarian mass [Table/Fig-6]. Out of 50 women 14 (28%) had score ≤ 2, five (10%) had score 3-4(equivocal) while 31(62%) were having score ≥ 5 [Table/Fig-7]. Women (5) having score of 3-4 were left out from statistical analysis considering them equivocal. Colour Doppler and spectral Doppler was done in all 50 women and they were divided accordingly. Out of 50 women 20(40%) were suggestive of malignancy while 30(60%) were suggestive of benign ovarian mass on Colour Doppler and Spectral Doppler study [Table/Fig-8].
B Mode morphology of ovarian masses
| Ovarian Mass | N = 50 |
|---|
| Unilocular Cystic | 10 (20%) |
| Multilocular Cystic | 10 (20%) |
| Complex Cystic | 29 (58%) |
| Solid | 01 (02%) |
Patients grouped according to B- Mode morphological score and histopathological diagnosis.
| B-Mode USG results | Diagnosis | Total |
|---|
| Malignant | Benign |
|---|
| Score ≥ 5 | 17 | 14 | 31 |
| Score ≤ 2 | 1 | 13 | 14 |
| Total | 18 | 27 | 45 |
Statistical Analysis:-Chi-square with Yates correction = 7.262, p-value = .007(<.05)
Sensitivity: 94.44% Specificity: 48.15% +ve predictive value: 54.84% –ve predictive value: 92.86%
Patients grouped according to (Colour + Spectral) Doppler and Histopathological diagnosis
| Colour Doppler + Spectral Doppler | Diagnosis | Total |
|---|
| Malignant | Benign |
|---|
| +ve | 17 | 3 | 20 |
| -ve | 3 | 27 | 30 |
| Total | 20 | 30 | 50 |
Statistical Analysis: Fisher’s exact test p-value = .0001(<.05)
Sensitivity- 85% Specificity- 90% +ve predictive value- 85% –ve predictive value- 90%
On analysing pre menopausal women and menopausal women with histopathological diagnosis p-value = 0.0001, which was statistically significant and suggest that menopausal women have more chances of having malignant ovarian masses [Table/Fig-4].
On analysing B- Mode morphological score and histopathological diagnosis sensitivity, specificity, positive predictive value and negative predictive value was 94.44%, 48.15%, 54.84% and 92.86%, respectively with p-value = 0.007, which was statistically significant and suggest that B-Mode USG had significant sensitivity and negative predictive value for ovarian masses [Table/Fig-7]. By these calculations diagnostic accuracy of B-Mode USG for ovarian masses comes to around 67%.
On analysing Doppler scan (Colour + Spectral) and Histopathological diagnosis sensitivity, specificity, positive predictive value and negative predictive value was 85%, 90%, 85% and 90%, respectively with p-value = 0.0001, which was statistically significant and suggest that Doppler scan had significant sensitivity, specificity, positive predictive value and negative predictive value for ovarian masses [Table/Fig-8]. By these calculations diagnostic accuracy of Doppler scan for ovarian masses was 88%.
Discussion
Ovarian carcinoma is a significant public health problem. Martin VR wrote in his article that anatomical and histological changes in ovaries during woman’s lifetime help explain why ovarian cancer is not readily detected in early stages [8]. The five-year survival rates are greater than 80% for stage IA or lB tumours and less than 5% at stage IV [9]. The significance of this problem has generated much interest in the evaluation of all aspects of ovarian cancer and, specifically, interest in the detection and characterization of ovarian masses by means of radiologic imaging.
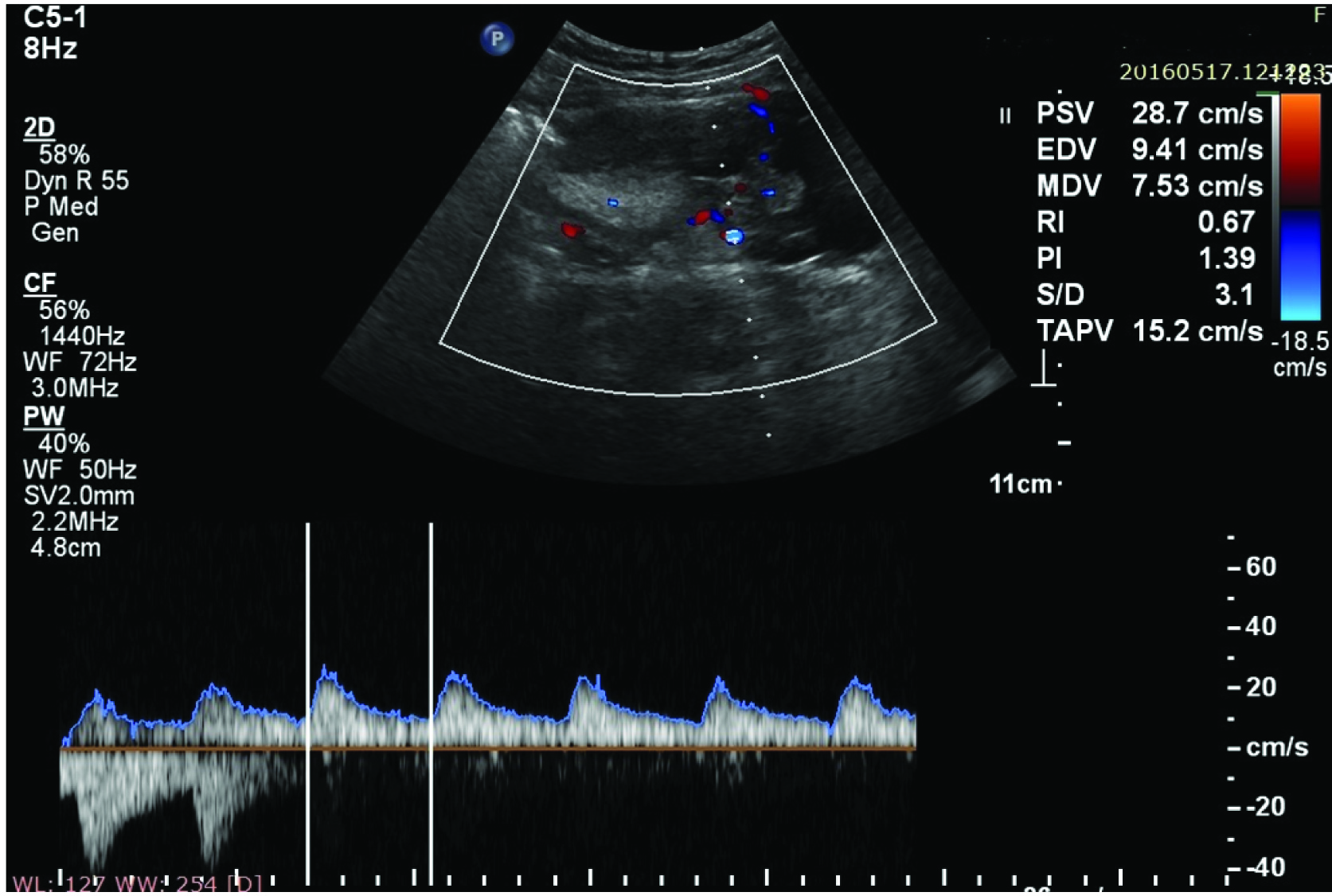
Ultrasonic signs on morphologic assessment of malignant ovarian tumours include multilocular or multiple cysts, thick or irregular septa or walls, poorly defined borders, mural nodules, solid components and echogenic elements. On colour Doppler presence of vascularity, central site of vessels, random vessel arrangement and abnormal vessel morphology were suggestive of malignant ovarian lesion. Spectral Doppler findings suggestive of malignancy are when Resistive Index (RI) value is ≤ 0.4 and Pulsatility Index (PI) value is ≤ 1.0 [10]. High resistive index and pulsatility index values are suggestive of benign pathology in adnexal masses [Table/Fig-9].
Adnexal mass showing high PI value and high RI value on Spectral Doppler scan
Ji In Choi et al., also proposed algorithm for differential diagnosis of complex solid and multicystic ovarian lesions on the basis of imaging features [11]. Our study suggests high risk of ovarian malignancy in post menopausal women. In this study 66.7% post menopausal women had ovarian malignancy compare to 8.7% pre menopausal women, which is also statistically significant. Ko-Hui Tung et al., also reported 61% postmenopausal women having ovarian cancer in their study [12]. Once a woman enters menopause, there is an expectation that the ovaries become inactive. While they do have a lower level of activity, they are still capable of producing cysts. Patricia G. Moorman et al., reported that premenopausal women were more likely to have tumours of low malignant potential [13]. A major hypothesis is that the hormonal changes during pregnancy lead to a “clearing” of transformed cells in the ovarian epithelium. In particular, high progesterone levels during pregnancy may have an apoptotic effect on transformed cells and this protective factor is lost after menopause.
In present study, most common benign lesions were inflammatory ovarian lesions and serous cystadenoma while 50% of malignant ovarian masses were serous carcinoma. In our study on B- Mode USG was most common ovarian lesions were complex cystic followed by unilocular and multilocular ovarian cysts.
Morphological indexing of the adnexal masses was also done by Sassone et al., based on the visualization of inner wall structure and wall thickness, septae, solid parts and echogenicity [14]. In our study, sensitivity and negative predictive value of B-Mode USG is more than 90%, which is also statistically significant. It suggests that this modality is highly accurate in diagnosing true positive patient among the women who have malignant ovarian masses. It also suggests that B-Mode USG is very accurate in diagnosing true negative patient among the women who have benign ovarian masses. Sharon M Stein et al., also reported high sensitivity (98%) and negative predictive value (99%) in their study [15].
In our study sensitivity and positive predictive value of Doppler scan (Colour + Spectral) was 85% each while specificity and negative predictive value was 90% each, which was also statistically significant. It suggests that this modality is overall more accurate in differentiating various ovarian neoplasms. Maden R et al., also reported specificity of 96.8% and positive predictive value of 95.2% in their study [16].
Potential pitfall of USG imaging for ovarian masses include inter observer variation, experience of radiologist and inability to compare previous USG imaging as it is real time modality. These can lead to diagnostic overcalls and undercalls [17].
Recently International Ovarian Tumour Analysis (IOTA) strategies and Risk of Malignancy Index (RMI) are two commonly used USG based system to diagnose ovarian masses. A Testa et al., conclude in their study that all IOTA strategies had excellent diagnostic performance in comparison with RMI [18]. While Sayasneh A et al., concluded that the test performance of IOTA prediction models and rules as well as the RMI was maintained in examiners with varying levels of training and experience [19]. Nunes et al., concluded that the IOTA protocol could be used in 76–89% of tumours and is an accurate test for the diagnosis of ovarian cancer [20].
Limitation
Study limitations are less number of patients and all sonography done by junior radiologist. As study was conducted in medical college so pathologists skill and experience might have variance reporting histopathology and cytology of ovarian masses.
Conclusion
Early stage diagnosis of an ovarian malignancy is the most important objective for better prognosis either in term of cure or post treatment long term disease free survival. USG and its different techniques are accepted as the primary imaging modality in the evaluation of ovarian masses. Post menopausal women are at higher risk compared to premenopausal women. B-Mode USG has significant sensitivity and negative predictive value for ovarian masses while Doppler scan has higher specificity. In present study, statistical analysis suggests Doppler Scan (Colour + Spectral) more accurate than B-Mode USG, but author is in view that optimal ovarian lesion characterization appears to be obtained through the combination of gray scale USG morphology and Doppler imaging information.
Statistical Analysis:- Chi-square with Yates correction = 15.06, p-value = .0001(<.05)
Statistical Analysis:-Chi-square with Yates correction = 7.262, p-value = .007(<.05)
Sensitivity: 94.44% Specificity: 48.15% +ve predictive value: 54.84% –ve predictive value: 92.86%
Statistical Analysis: Fisher’s exact test p-value = .0001(<.05)
Sensitivity- 85% Specificity- 90% +ve predictive value- 85% –ve predictive value- 90%