Metabolic state of the bone in the patients having thyroid disorders can be specifically assessed to a certain extent by measurement of bone specific alkaline phosphatase (Ostase). Thyroid hormones play pivotal role in regulation of various types of metabolic activities. Disorder in thyroid function may affect bone metabolism which may in turn lead to disorders in the skeletal growth, peak bone mass acquisition and maintenance of bone mass. Hypothyroidism compromises-normal bone formation and results in slowing of linear growth [1–4]. Thyrotoxicosis leads to growth acceleration, diminution of bone mass and advance in bone age. Biochemical bone markers like serum bone specific ALP (Ostase) level are sensitive to changes in bone turnover, as ALP is a product of osteoblastic activity [5–9]. Measurement of ostase in the serum provides a more specific assessment of the metabolic status of bone in normal and pathological conditions. The measurement of serum ostase has several advantages over the measurement of other bone parameter, because of its relatively long half-life invivo (1 to 3 days), it is relatively unaffected by diurnal variation. Also, ostase is more stable in vitro and does not require special specimen handling. Ostase estimation is more useful in individuals with impaired renal function because it is not cleared by glomerular filtration [10].
Ostase is not routinely advised or done in hyperthyroid and hypothyroid cases as a standard co-morbidity evaluation protocol.
The high prevalence of thyroid disorders and its potential effect on long-term Bone Mineral Density (BMD) and overall bone health of the patients of this region of north eastern India, where standard health care facility is not adequate, necessitates the monitoring and follow-up of such cases with a sensitive, reliable, cheap and easily available assay where serum ostase can be a proper fit.
The present study was aimed primarily to find out the correlation of serum ostase level in hyper and hypothyroid cases and also to study the validity of routine estimation of serum ostase in hyper and hypothyroid cases so as to monitor the base level bone health on presentation in these cases. This may in turn help in prevention, management and follow-up of bone related co-morbidity in such cases.
Materials and Methods
This cross-sectional study was conducted in the Department of Biochemistry in North Eastern Indira Gandhi Regional Institute of Health and Medical Sciences (NEIGRIHMS) after obtaining ethical clearance from the institute. The study was conducted for two years from April 2013 to March 2015. The number of cases was 74 and controls were 20.
In 74 patients with disorders of thyroid function serum ostase levels were estimated. Among them, 51 were female and 23 were male. A total of 39 patients were hypothyroid, 31 were hyperthyroid & 4 patients had subclinical hyperthyroidism. None of the female patients were pregnant. Postmenopausal osteoporotic women were not on any hormonal replacement therapy at the time of study.
Twenty consenting healthy individuals (11 male and 9 female; Age range 20-35 years) with normal thyroid function were taken as a control group and the serum ostase levels were estimated. They were not receiving any type of medication at the time of blood sampling.
Specimen Collection and Preparation: 4ml blood was collected in vacutainer, allowed to clot completely before centrifugation and centrifused (1000g, 10min, 200C). The supernatant serum was removed for analysis. Serum ostase level, Thyroid Stimulating Hormone (TSH), FT3 (Free 3,5,3/-triiodothyronine), FT4 (Free 3,5,3/,5/ tetra- iodothyronine) levels were estimated by chemiluminescent technique. Instrument used was Beckman- coulter Access2.5μL of serum was required for estimation. Residual fibrin and cellular matter was removed prior to analysis.
Principles of the procedure: The Access2 Ostase assay is a one-step immune-enzymatic assay. A mouse monoclonal antibody specific to Bone Specific Alkaline Phosphatase (BAP) was added to a reaction vessel with paramagnetic particles coated with goat anti-mouse polyclonal antibody. Calibrators, controls and samples containing BAP were added to the coated particles and bound to the anti-BAP monoclonal antibody. Following the formation of a solid phase/capture antibody/BAP complex, materials bound to the solid phase are held in a magnetic field while unbound materials are washed away. Then, the chemiluminescent substrate Lumi-Phos* 530 was added to the vessel and light generated by the reaction was measured with a luminometer. The light production is directly proportional to the concentration of BAP in the sample. The amount of analyte in the sample was determined from a stored, multi-point calibration curve [11].
Results
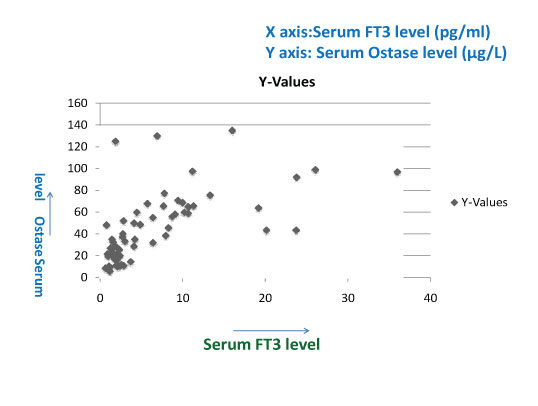
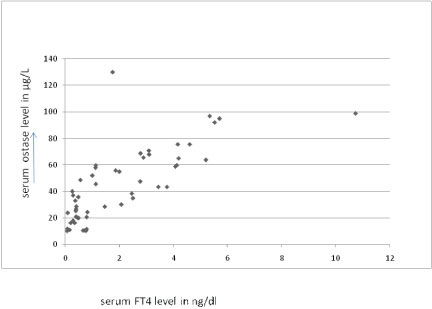
The serum ostase level was significantly raised in hyperthyroid patients showing positive correlation between serum FT3, FT4 and serum ostase level in patients with various thyroid disorders [Table/Fig-1,2,3 and 4] and negative correlation between serum TSH level and serum Ostase level [Table/Fig-5].
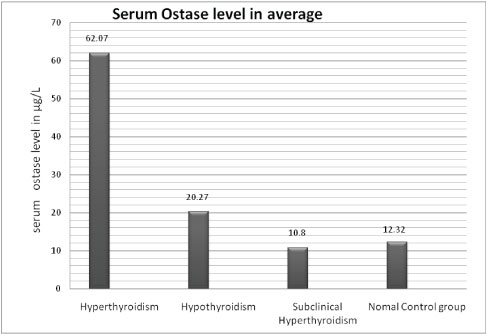
Showing average value of serum ostase level in normal control group & various types of thyroid disorders.
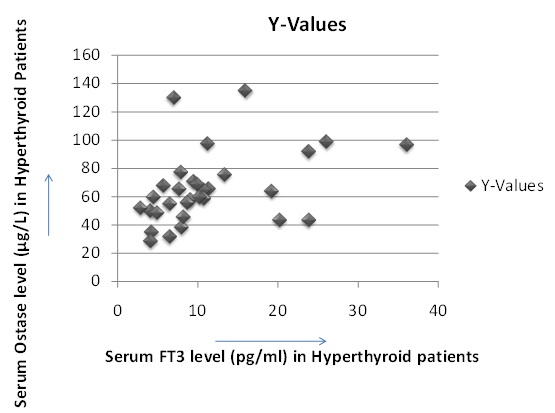
Showing positive correlation between serum FT3 & serum Ostase level in hyperthyroid patients (correlation coefficient 0.4632).
Showing positive correlation between serum FT3 & serum Ostase level in thyroid disorders (correlation coefficient 0.7364).
Showing positive correlation between serum FT4 & serum Ostase level in thyroid disorder. (Correlation coefficient 0.7435).
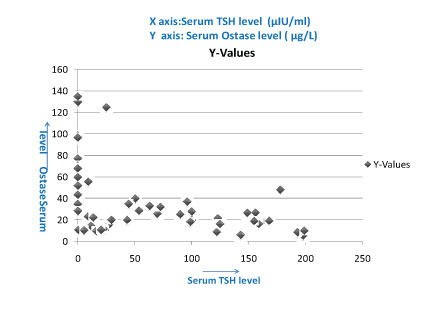
Showing negative correlation between serum TSH level & serum Ostase level, (correlation coefficient - 0.5075).
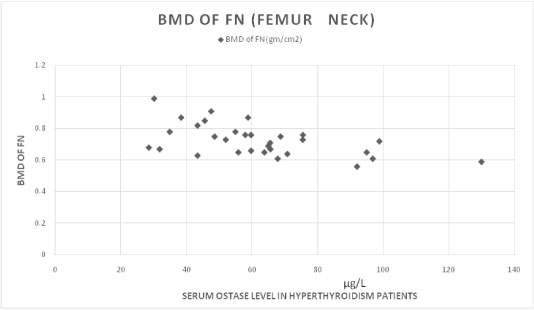
Serum ostase levels and BMD showed a negative correlation in hyperthyroid patients [Table/Fig-6]. [Table/Fig-7] showing the correlation between ostase levels & bone mineral densities in normal control individuals.
Showing negative correlation between serum Ostase level & BMD in hyperthyroid cases. (correlation coefficient -0.544).
Showing correlation between BMD level & serum ostase level in normal control gp.

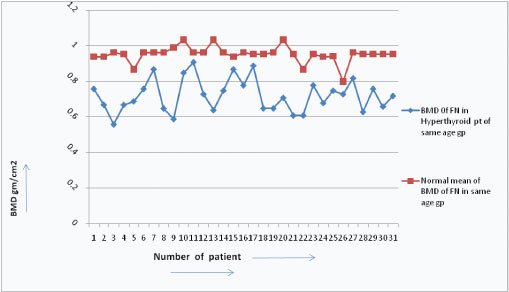
BMD in hyperthyroid patients were found decreased than the BMD of normal persons of the same age group [Table/Fig-8].
Showing comparision of BMD level between normal & hyperthyroidism patients in same age.
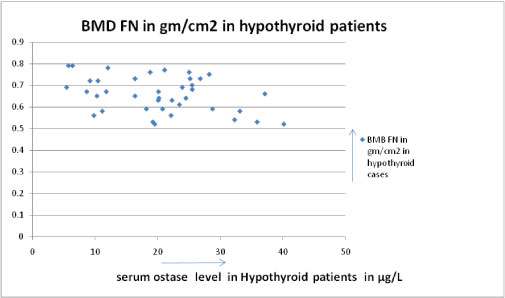
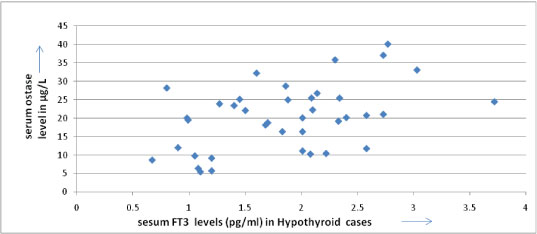
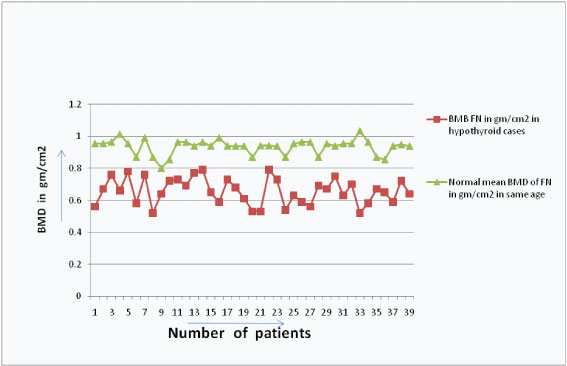
The serum ostase and bone mineral density showed a negative correlation in hypothyroid patients [Table/Fig-9] but positive correlation between serum FT3 and serum Ostase level in hypothyroid patients [Table/Fig-10]. The BMD were also found to be decreased than the BMD of normal persons of the same age group in hypothyroid patients [Table/Fig-11].
Showing negative correlation between serum Ostase level & BMD in hypothyroid cases. (correlation coefficient -0.382).
Showing positive correlation between serum FT3 & serum Ostase level in hypothyroid patients.(correlation coefficient 0.461).
Showing comparison of BMD level between normal persons and hypothyroidism patients in same age gp.
Discussion
The results of our cross-sectional study were similar with the findings of previous studies which reported an increased ostase level along with decreased BMD in hyperthyroid cases [12–14]. In the control group, all the individuals had a normal thyroid function (i.e. normal serum TSH, FT3, FT4 level) and their serum ostase level also was within the normal range (average 12.3245μg/L ±1.034 SD).
{Normal range of serum FT3 level is 2.50-3.90pg/ml,FT4 level is 0.61-1.12 ng/dl,TSH level is 0.34-4.65μIU/ml, Ostase level is 8-16.6μg/L}.
In 31 hyperthyroid patients we found high serum FT3 level (average FT3 level is 11.3 pg/ml ±SD 7.7) and high serum ostase level (average ostase level is 62.07μg/L ± SD 22.82). We found a positive correlation between their FT3 and Ostase level (correlation coefficient is 0.4632), p-value is significant<.01. We also measured BMD (Bone Mineral Density) by DEXA scan of these 31 cases and their BMD values were decreased than normal range of same age group, moreover their BMD values and Serum Ostase level showing negative correlation (correlation coefficient is - 0.544), p-value is significant <.01. It is due to increased osteoblastic activity and abnormal bone remodeling in hyperthyroidism. Bone remodeling is a dynamic process and characterized by coupling between resorption and formation. The bone architecture and strength are maintained by a balanced process of remodelling, which involves recruitment of osteoclast and osteoblasts. The sequence of events in remodelling, i.e., activation – resorption – formation (ARF sequence), is most easily demonstrated in cortical bone.
FT3 is responsible for major actions of thyroid hormones. In osteoblasts, FT3 regulates cell differentiation and bone matrix synthesis and degradation, thereby acting as an essential regulator of bone mineralization and strength. It binds to its nuclear receptors TRα1 and TRβ1 in osteoblast that regulates gene transcription via interaction with thyroid hormone response element of specific genes allowing production of ostase [15]. Recently, non-genomic actions of T3 and T4 have been described [16]. Local tissue availability of T3 seems to be regulated by type 2 and 3 deiodinase [17].
Molecular mechanism of action of T3 on bone is very intricate and poorly understood, however lot of theories were provided for T3’s action in molecular level. T3 is involved in local signalling pathways by stimulating osteoblast responses to IGF1-I (Insulin Like Growth Factor-l), PTH (parathyroid hormone) and fibroblast growth factors. T3 regulates the rate of chondrocyte differentiation by modifying the set point of the Indian hedgehog (Ihh) – Bone Morphogenetic Protein (BMP) – PTHrP (parathyroid hormone related peptide) long feedback loop [18]. Hypothyroidism is marked by increased PTHrP expression and impaired hypertrophic chondrocyte differentiation [1]. In hyperthyroidism, reduced expression of PTHrP associated with augmentation of BMP enhances hypertrophic chondrocyte differentiation [19]. In addition, T3 can promote hypertrophic differentiation of chondrocyte by induction of cyclin-dependent kinase inhibitors to regulate the G1–S cell cycle checkpoint [20]. T3 mediates osteoclastic bone resorption through activation of osteoblasts, which then release receptor activator for NF-κB ligand (RANKL), a member of the TNF cytokine family. RANKL is a ligand for osteoprotegerin, a cytokine that regulates osteoclastic differentiation, and functions as a key factor for osteoclast differentiation and activation by inhibiting osteoclasts apoptosis [21]. T3 stimulates osteoblast activity both directly and indirectly via numerous growth factors and cytokines. By contrast, osteoclasts only resorb bone in response to T3 in the presence of co- cultured osteoblasts, indicating that osteoblasts are the primary T3 target cells that regulate bone turnover in response to T3.
In 39 hypothyroid patients we found low serum FT3 level (Average FT3 is 1.85 pg/ml± SD 6.9) and their ostase levels were not very high (average ostase level is 20.27μg/L ± SD 8.9). Both the parameters showed a positive correlation (correlation coefficient is 0.461), p-value was significant < 0.01; their BMD values were decreased than normal range of same age group; moreover their BMD values & Serum Ostase level showed a negative correlation (correlation coefficient is - 0.382), p-value was significant < 0.01. Similar findings are observed in previous studies [22,23].
In 4 subclinical hyperthyroid patient serum FT3 level average was 2.54pg/ml±SD 0.44 and serum average ostase level was 10.83μg/L±0.12. Both parameters showed a positive correlation (correlation coefficient was 0.75). Subclinical hyperthyroidism is defined as subnormal serum TSH with normal serum free thyroid hormones without signs or symptoms of thyrotoxicosis. Subclinical hyperthyroidism prevalence in the US [4,24], by definition, patients suffering from subclinical hyperthyroidism should not have any clinical abnormalities associated with thyrotoxicosis [25]. However, a reduced bone mass was reported among postmenopausal subclinical hyperthyroidism patients [26]. In both, clinical and subclinical hyperthyroidism, elevation of markers of bone turnover and decreased BMD have been reported [22,27,28].
In this study, we observed negative correlation between serum TSH and ostase level in both hyper and hypothyroidism, correlation coefficient is -0.5075 [Table/Fig-5]. Recent studies proposed that TSH acts as a direct negative regulator of bone remodeling, highlighting the importance of integrity of the hypothalamo -pituitary-thyroid axis [4]. In several recent studies it has also been reported that Thyrotropin (TSH) inhibits bone turnover by a direct interaction with the specific receptor expressed on bone cells. In experimental animals, the reduction in expression of the TSH receptor leads to the development of osteoporosis, providing evidence for a suppressing effect of this hormone on bone turnover [29]. To our knowledge, there are no studies showing similar effects of TSH in humans [29]. A physiological activity of TSH on bone cells seems to be unlikely because of the weak expression of the TSH receptor in human osteoblasts [30]. However, it is unclear whether TSH suppression or TSH increase, as they occur in thyrotoxicosis and hypothyroidism, may have any effect on bone metabolism. In both pathological conditions, in fact, the abnormal serum thyroid hormone levels hinder evaluation of the specific effects of TSH on bone function [31–35].
Limitation
The only limitation found during the study was inability to include the estimation of serum osteocalcin & serum PICP (Caboxy terminal Propeptide of type I Procollagn) along with serum ostase due to lack of assessing facilities of the instrument. These two parameters are also strong biochemical markers of bone osteoblastic activity and this leaves a scope of future studies on this topic along with these two parameters.
Conclusion
It was observed that the serum ostase level was elevated in almost all cases of hyperthyroidism along with decrease in Bone Mineral Density (BMD). Serum ostase level was found directly proportional to the serum FT3 level.
A routine estimation of serum ostase level in hyperthyroid cases will help in proper monitoring of decrease bone turnover as indicated by increased serum ostase level. Besides, the estimation of serum ostase level in hyperthyroid cases was found to be valid in this study which can turn to be a very sensitive and important guiding parameter to the treating physician to formulate necessary protocols and guidelines for prophylaxis, treatment and to monitor the response to therapy in cases of reduced bone turnover related to hyperthyroid state. However, no significant relation of serum ostase activity was noted in any hypothyroidism or any thyroid related disease in this study, probably due to the less number of studied cases. Further research is needed to study the impact of hyperthyroidism, subclinical hyperthyroidism and hypothyroidism on serum ostase level and to evaluate the changes of bone mineral density and fracture risk among those patients.