Chyluria is described as the passage of milky urine due to the presence of chyle and is usually due to lymphatic filariasis [1]. Approximately 1,100 million people across the globe are living in endemic region for lymphatic filariasis and exposed to risk of infection. In the year 2000, WHO started a worldwide campaign to eliminate filariasis. India contributes about 40% of total global burden and accounts for nearly 50%of the people at risk of filarial infection and the province of Uttar Pradesh has the highest prevalence, with 7 million affected people [2–4]. Chyluria occurs in up to 10% of patients with filariasis [5]. Wuchereria bancrofti responsible for ~90% cases of filariasis worldwide and over 99% of cases of filariasis in India [6].
Several genes and their alteration contribute to disease susceptibility and outcomes especially in cases of autoimmune disorders and malignancies [7]. Investigators have tried to evaluate the role of genetic susceptibility to infectious disease also [8]. However, data looking at genetic predisposition for filariasis is scanty and controversial. To the best of our knowledge, only two studies have looked into this aspect till date. Moreover, these studies are more than 10-year-old. Choi et al., found an association between 24bp duplication mutation in exon 10 of the chitotriosidase gene (CHIT1) and susceptibility to filarial infection in south-Indian population [9] while Hise et al., in their work, did not find any significant correlation with infection status or disease phenotype [10].
Materials and Methods
This case-control study, carried out between March 2013 and December 2015, was approved by the Institutional Ethics Committee (Reference code number 5534/R). cell-13. A total of 168 patients presented with chyluria during the study period of which 155 cases were found positive for FC. After obtaining written informed consent from patients, data was collected using a standardized questionnaire. Patients older than 18-year-old with FC, confirmed by specific diagnostic tests were enrolled. Subjects with, whitish-cloudy urine, non-parasitic chyluria, malignancy, pregnancy, renal failure and uncontrolled diabetes were excluded. Filarial aetiology of chyluria was confirmed using Giemsa stained thick and thin smear examination followed by DEC provocative test [12,13], immune-cromatographic card test (ICT) (BinaxNOW® filariasis, Alere North America, Orlando, USA) [14] and IgG/IgM combo rapid antibody test [15]. The patients who were found positive by either of these tests were considered for FC. For comparison, control subjects without any symptoms or signs of lymphatic filariasis, confirmed by negative ICT and IgG/IgM combo rapid antibody test, were enrolled after obtaining consent. The controls chosen were matched based on age, sex and geographical region to the cases. The parameters collected included demographic data (age, sex, and ethnicity), details of chyluria like duration, grading of chyluria, number of episodes, chylous clot retention and details on investigations like haemoglobin, urinary parameters like range of urine triglycerides and cholesterol levels were extracted from the records. After collecting the above details, 5ml of blood sample was obtained from each subject that was aspirated into an EDTA coated vial and stored at -20°C to perform diagnostic tests, DNA isolation and polymorphism study.
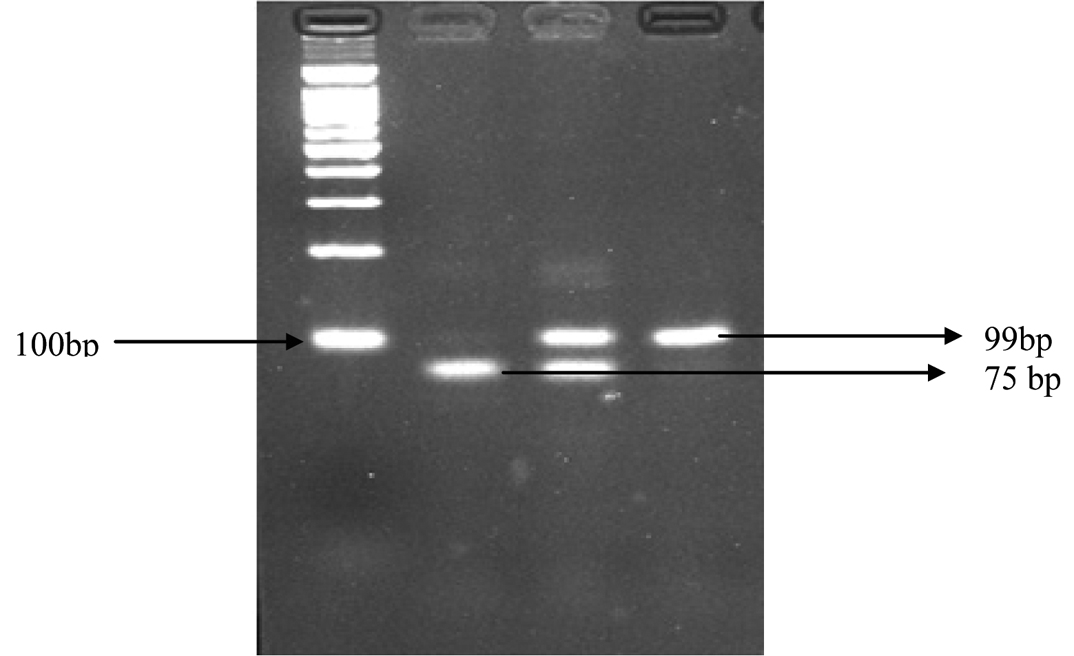
Single Nucleotide Polymorphism in CHIT1 Gene [Table/Fig-1]: Genomic DNA was extracted from whole blood using commercially available DNA isolation kit (Quick- g DNATM, USA) as per manufactures’ protocol. 100-ng of DNA was used as a template in subsequent PCR reactions. The duplication mutation analysis was performed using specific CHIT1 primers (F: 5’AGCTATTCTGAAGCAGAAG3’ and R: 5’GGAGAAGCCGGCAAAGTC3’). The product was amplified at 94°C for 5 minutes, at 94°C for 40 second, 55°C for 38 seconds, at 72°C for 45 seconds and 72°C for 7 minutes. The amplified PCR product was separated on 3%- agrose gel in 1x TBE buffer and visualized by ethidium bromide staining. Gels were viewed using the Bio imaging system. The size of wild type product was 75 base pair whereas the mutant product was 99 base pairs due to the mutant allele containing 24 base pair duplication in exon10 [16].
Lane M: 100 bp ladder, Lane1: homozygous HH (75bp), Lane 2: heterozygous HT (99bp and 75bp), and Lane 3 : homozygous TT (99bp).
Statistical Analysis
The results are presented in mean±SD and percentages. The Chi-square/Fisher exact test was used to compare the dichotomous/categorical variables between cases and controls. The unpaired t-test was used to compare the age distribution between cases and controls. The univariate and multivariate binary logistic regression was used to find the risk of genotypes. The adjusted and unadjusted Odds Ratio (OR) with its 95% Confidence Intervals (CI) was calculated. The p-value<0.05 was considered significant. All the analysis was carried out on SPSS 16.0 version (Chicago, Inc., USA).
Results
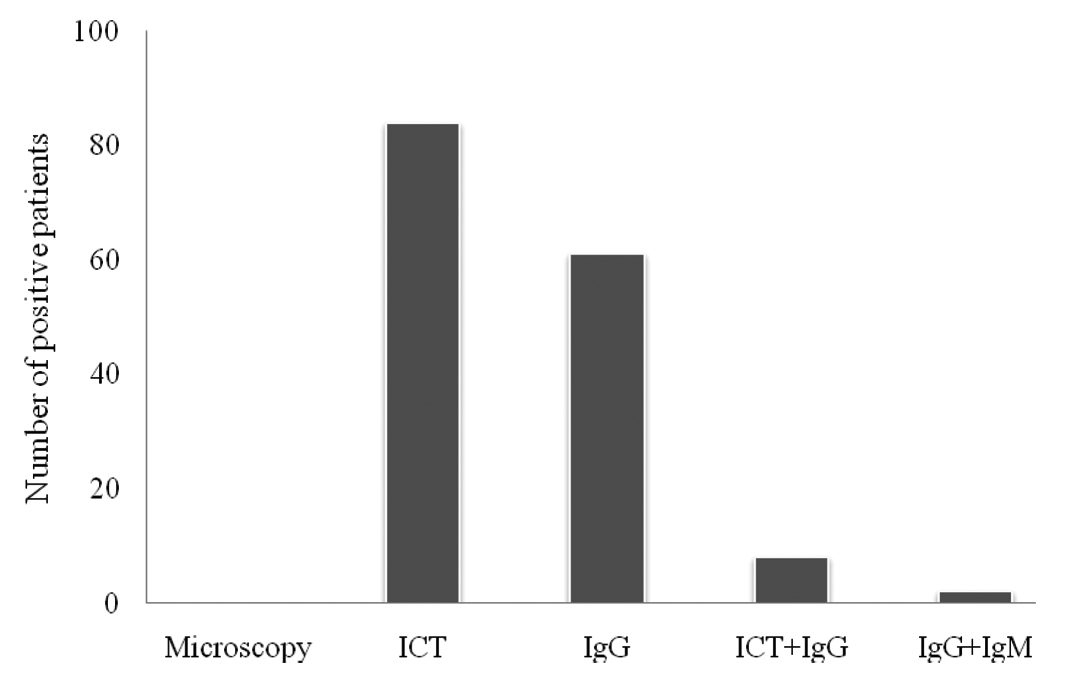
Demographic profile of patients and controls is shown in [Table/Fig-2]. Clinical presentation of patients is shown in [Table/Fig-3]. Of the 155 patients tested to confirm filarial aetiology, 84 patients were positive with ICT only, 61with IgG only, 8 with both ICT and IgG and 2 patients were positive for both IgG and IgM [Table/Fig-4]. CHIT1 genotype distribution of FC patients diagnosed with various laboratory tests, as mentioned in methods section is shown in [Table/Fig-5]. The patients diagnosed by ICT had more Heterozygocity (HT) than those diagnosed with either IgG or IgM test.
Demographic characteristics of subjects.
| Cases (n=155)mean±SD | Control (n=155) mean±SD | p-value |
|---|
| Age (years) |
| 18-29 | 39(25.16%) | 65(41.9%) | 0.09a |
| 30-39 | 40(25.8%) | 36(23.2%) |
| 40-49 | 41(26.4%) | 31(20%) |
| 50-59 | 25(16.1%) | 16(10.3%) |
| >59 | 10(6.45%) | 07(4.5%) |
| 38.25±12.09 | 35.45±12.53 |
| Gender |
| Male | 110(71.0%) | 113(72.9%) | 0.70b |
| Female | 45(29.03%) | 42 (27.09%) |
| Residence |
| Rural | 106(68.38%) | 95(61.26%) | 0.19b |
| Urban | 49(31.61%) | 60(38.70%) |
aUnpaired t-test, bChi-square test
Clinical profile of FC patients.
| Clinical characteristics | Cases (Mean±SD) |
|---|
| Total disease duration of chyluria (months) | 62.81±60.83 |
| Number of episodes | 2.54±1.11 |
| Duration of current episode (days) | 10.64±7.28 |
| Urine TGS level g/dl | 261.36±253.61 |
| Urine Cholesterolg/dl | 24.51±39.92 |
| Haemoglobin g/dl | 11.55±1.58 |
Laboratory tests among FC patients.
Frequency of CHIT1 genotype with diagnosis pattern of FC patients.
| Genotype distribution |
|---|
| HH | HT | TT | HT+TT |
|---|
| ICT | 08(5.1%) | 67(43.2%) | 09(5.8%) | 76(49.03%) |
| IgG | 09(5.8%) | 48(30.9%) | 04(2.5%) | 52(33.54%) |
| ICT+IgG | 01(0.6%) | 07(4.5%) | 00(0%) | 07(4.5%) |
| IgG+IgM | 00(0%) | 02(1.2%) | 00(0%) | 02(1.2%) |
On comparison of the genotypic frequencies for 24 bp duplication, the Homozygous genotype (HH) was found to be more prevalent among controls (18.7%) than among FC patients (10.3%). However, heterozygous genotype (HT) was significantly more prevalent among FC patients (81.3%) than controls (75.5%), (p=0.04). Which conferred the increased risk towards filarial patients with OR 1.95, 95% CI (1.01–3.77). Also, the combination of HT and homozygous mutant TT genotype revealed a significant p-value 0.03 with OR 2.0 and 95% CI (1.03-3.85). However, the frequency of TT genotype was slightly higher in the FC subjects (8.4%) than controls (5.8%), with OR 2.61, 95% CI (0.91-7.45), and p = 0.07 [Table/Fig-6].
CHIT1 genotypic and allelic frequencies among cases and controls
| Cases(n=155) | Control(n=155) | Unadjusted OR (95%CI) | p-value |
|---|
| No. | % | No. | % |
|---|
| Genotype frequencies |
| HH | 16 | 10.3 | 29 | 18.7 | 1.00 (Ref.) | |
| HT | 126 | 81.3 | 117 | 75.5 | 1.95 (1.01-3.77) | 0.04* |
| TT | 13 | 8.4 | 9 | 5.8 | 2.61 (0.91-7.45) | 0.07 |
| HT+TT | 139 | 89.7 | 126 | 81.3 | 2.00 (1.03-3.85) | 0.03* |
| Allele frequencies |
| HH | 158 | 51.0 | 175 | 56.5 | 1.00 (Ref.) | |
| TT | 152 | 49.0 | 135 | 43.5 | 0.80 (0.58-1.10) | 0.19 |
OR-Odds ratio, N- number, CI-Confidence interval, Ref.: Reference, *P- <0.05
Allelic frequencies for both the groups also observed wild allele (HH) was slightly more prevalent among the control subjects (56.5%) than in the filarial patients (51.0%), while the mutant allele (TT) was more prevalent among the FC patients (49.0%) compared to the controls (43.5%), {OR 0.80, 95% CI (0.58-1.10} and p = 0.19 [Table/Fig-6].
Discussion
Filariasis is endemic in northern part of India, where our Medical University is located. The entire population in this area is exposed to mosquitoes but only few individuals get affected clinically, we planned this study to look for genetic factors that might predispose an individual to FC. Therefore, individuals from the same geographical region as controls were recruited.
Although, studies looking at the genetic aspect of various diseases are common, unfortunately filariasis has escaped the attention of investigators. To our surprise we could find only two studies that have looked into this aspect of the disease. As both these studies included CHIT1 gene with conflicting results, we also evaluated the same gene [9,10].
In the previous studies, patient recruitment was heterogeneous with patients having varying manifestation like, elephantiasis, hydrocele, etc. being included. For current study we included only patients suffering with chyluria attending urology OPD.
The CHIT1 gene, also known as Chitinase 1 or Chitotriosidase1, is a 50-kDa mammalian chitotriosidase enzyme that is detected in serum of both healthy and diseased individuals [17]. Enzymatically-active chitinases cleave chitin, which is present in diverse organisms like cell wall of fungus, exoskeleton of mites and arthropods, lining of the insect gut and the microfilarial sheath of parasitic nematodes [18,19]. These pathogens use chitin for their protection against the animal or plant host immune machinery and absence of this chitin may lead to the death of the pathogen. Therefore, chitinase production is very important in the life cycle of any fungi or parasite for its survival in the host [20]. The β-1,4-glycosidic bonds of chitin is broken down by chitinase that releases N-acetylglucosamine dimers that are then acted upon by N-acetylglucosaminidase [21]. CHIT1 which is produced, stored and secreted by neutrophils and macrophages plays a crucial role as an innate immunity defense mechanism against chitin-containing pathogens. The human CHIT-1 gene consists of 12 exons localized on chromosome 1q31–q32 [22]. Duplication of 24bp in exon 10 of CHIT1 gene, found by sequencing method was associated with a recessive mutation. This duplication introduced a 30 splice site in the coding region of CHIT1 that resulted in deletion of subsequent 87 nucleotides. Loss of these nucleotides introduces a change of amino acid residues from 344 to 372 in the polypeptide chain of chitotriosidase leading to the inactivation of chitinolytic activity [16]. Inactivation of this enzyme leads to the enhanced survival of pathogen which may play a role in the occurrence of FC.
In our series the male (110) to female (45) ratio was 2.4:1 which is similar to most studies reported in the literature. In the current study, urinary Triglycerides (TGs) levels ranged between 0.7 and 1193.5g/dl (normal range <0.15g/dl) and cholesterol levels ranged between 0.2 and 369g/dl (normal range <0.2g/dl) in FC patients. Several studies have shown the significance of urinary TGs in evaluating chyluria [23].
Demonstration of microfilaria in blood gives conclusive and direct evidence of the aetiology of filariasis. DEC-provocative test is 80% efficacious in demonstrating microfilaremia, but in our patients none had. This may be because of absence of microfilariae in chronic manifestations of filariasis or parasitic load have been low. In current study more than half the patients were found positive for circulatory filarial antigen test which could be due to active infection of Wuchereria bancrofti. It is possible that each new episode of chyluria is induced by bite of an infected mosquito producing antigenaemia in the host. Of the total 61 patients were positive by IgG only, this may be because of the persisting circulating antibodies after the clearance of parasites or may be due to repeated exposure to the parasite. Bal et al., also reported that in endemic patients, level of antibodies increase due to lack of antigenemia [24]. Therefore, the ICT appears to be useful in predicting the filarial aetiology of chyluria.
In our study, we included patients who were diagnosed for filarial aetiology, either positive with ICT or IgG test. On the other hand, Choi et al., reported IgG titre to be positive in all the three groups of subjects with levels ranging from 137.6 in microfilariae positive patients, 67.1 in chronic pathology group and 36.8 in controls [9]. Similarly, they report a group of patients who were asymptomatic for filariasis but they were microfilaria and antigen positive. We have only considered controls as those participants who had no manifestation of filariasis and also had negative ICT and IgG levels.
We investigated the association between 24bp duplication of human CHIT1 gene in patients of FC. Our findings showed significant association between the (HT) heterozygous genotype (p=0.04) and combination of both the (HT+TT) heterozygous and mutant genotype (p=0.03) with susceptibility to FC. However, no association was found between recessive mutation TT and susceptibility to FC.
In patients who tested positive on ICT test the frequency of heterozygous (HT) condition was more (43.2%) than other tests. This may be due to the higher number of patients found positive by ICT test.
The previous studies have presented mixed reports on the association of CHIT1 gene polymorphisms with other manifestation of filariasis. For example, a study conducted in southern part of India reported 24bp -duplication in CHIT1 gene and susceptibility to filarial infection [9]. Another study conducted in Papua New Guinea looking at CHIT1 gene polymorphism reported non-significant association [10]. The differences in the disease association might be because of different genetic makeup associated with diverse ethnicity.
FC group has lower frequency of wild homozygocity (10.3% vs. 18.7%) and higher frequency of heterozygous (81.3%vs.75.5%) compared to control group. These results, however, are contradicted by Choi et al., [9]. Additionally, this group found an increased risk for disease susceptibility in patients with wild homozygous genotype (p=0.013). The difference of results between the two studies might be because of two reasons, firstly, relatively small number of uninfected individuals was examined in their study and secondly the diseases manifestations in both studies are different. In their study, patients presented with chronic lymphatic obstruction like elephantiasis or hydrocele. Also, as highlighted, there is a difference in the inclusion criteria for controls as we included subjects were negative for ICT, IgG and other tests as control.
Limitation
There are many limitations to our study too. We have included only patients of chyluria and patients with other manifestations of filariasis like hydrocele, lower limb lymphaedema, etc. were not included. Similarly, the sample size could have been larger. Also, many in control group can still develop the disease at a later date.
Conclusion
This is the first such report examining the polymorphism of CHIT1 gene in patients with FC in Northern India. Our results show significant association of filarial chyluria in heterozygous genotype. More studies are required to understand the genetic polymorphism with filarial chyluria in larger context. We have not correlated the genetic abnormalities with the clinical features of chyluria. However, we are presently evaluating our data for clinical correlation.
aUnpaired t-test, bChi-square test
OR-Odds ratio, N- number, CI-Confidence interval, Ref.: Reference, *P- <0.05