Enterococci are common commensals which inhabit the gut of human and animals and in recent decades, have emerged as one of the major causes of nosocomial infections [1]. Vancomycin has been used extensively for the treatment of serious infections due to Enterococci. However, after the first isolate of Vancomycin Resistant Enterococci (VRE) was reported in Europe in1986, there has been a steady increase in the number of VRE infections across the globe [1]. In hospitals, Intensive Care Units (ICU’s) are the major reservoirs for antibiotic resistant organisms including VRE. In healthy individuals colonization with VRE is less than 1% but its prevalence is much higher among patients admitted in ICUs [2]. Gut colonization predisposes to subsequent infection resulting in increased cost and morbidity [2,3]. A study from USA described 34 fold increase in the prevalence of VRE colonization in the ICUs between 1989-93 and also reported higher prevalence rate in hospitals with more than 500 beds [4].
A number of risk factors have been associated with VRE colonization in ICU’s, the most important ones being extended hospital stay, abdominal surgery and exposure to various antibiotics such as vancomycin, third generation cephalosporins and metronidazole [5–7].
In the present study we determined the rates of VRE colonization among patients admitted in Medical Intensive Care Unit (MICU) and the various risk factors which were associated with VRE colonization. To the best of our knowledge, this is the first report from India describing the VRE colonization rates in ICU patients.
Materials and Methods
This was a prospective study carried out over a period of 18 months from September 2013 to February 2015 after obtaining approval from the institute scientific advisory and human ethics committees. A 16 bedded MICU was selected for sample collection. Rectal swabs were collected after 48 hours of ICU admission from a total of 302 adult patients (156 Male and 146 Female). Readmitted patients to MICU were excluded from the study. Additionally samples were collected every 48 hours from 32 patients whose initial VRE colonization was negative. All the negative patients were not included due to financial and time constraints. The samples were processed within two hours of collection and inoculated on to Bile Esculin Sodium Azide Agar (BEA) containing vancomycin 6mg/l, incubated at 37°C for 24-72 hours [7]. Pinpointed black colonies were presumptively identified as Enterococci and were identified up to species level based on Facklam and Collin standard biochemical tests [8]. The Minimum Inhibitory Concentration (MIC) of vancomycin and teicoplanin was determined for all the enterococcal isolates grown on BEA by agar dilution method, with a MIC of ≥32 mg/L for both vancomycin and teicoplanin being considered as resistant [9]. The susceptibility of the isolates to other antibiotics was also recorded.
The demographic and clinical details of the patient such as age, sex, medical history, clinical diagnosis and co-morbidities such as diabetes, prior hospital admission, date of present admission to hospital and ICU and details of antibiotics administered were documented in a structured proforma. The patients were also followed up for evidence of laboratory confirmed VRE infections during their entire hospital stay.
Molecular detection of vancomycin resistance
PCR for vancomycin resistance genes (vanA, vanB, vanC1, van C2/C3) was performed in all the phenotypically vancomycin resistant isolates using published primers as given in [Table/Fig-1] [10].
Primer sequences used in PCR for vancomycin resistance genes [10].
| Target gene | Primer sequence | Amplicon size |
|---|
| vanA | A1 5’-GGGAAAACGACAATTGC-3’ | 732 bp |
| A2 5’-GTACAATGCGGCCGTTA-3’ |
| vanB | B1 5’-ATGGGAAGCCGATAGTC-3’ | 635 bp |
| B2 5’-GATTTCGTTCCTCGACC-3’ |
| vanC1 | C-1 C1 5’-GGTATCAAGGAAACCTC-3’ | 822 bp |
| C2 5’-CTTCCGCCATCATAGCT-3’ |
| vanC2/C3 | D1 5-CTCCTACGATTCTCTTG-3 | 437 bp |
| D2 5’-CGAGCAAGACCTTTAAG-3’ |
Extraction of DNA from the bacterial colonies was done using DNA extraction kit (Mericon DNA Bacteria Plus Kit, Qiagen) as per the manufacturer’s instructions. Amplification of the DNA was carried out in Mastercycler nexus (Eppendorf) with initial denaturation at 94°C, for 2 min and 30 cycles of denaturation at 94°C for 1 min, annealing temperature at 54°C for 1 min and extension at 72°C for 1min followed by final extension at 72°C for 10 min. The PCR product was analysed on 1.5% agarose gel. DNA ladder 100bp and 1kb was used to compare the size of the PCR amplified product. Electrophoresis was done at 100 V for one hour and gel was viewed under Molecular Imager Gel Doc XR + System (Bio-Rad Laboratories).
Statistical Analysis
Data was analysed using SPSS 20.0 version software. The outcome of colonization with VRE was expressed as binary categorical variable. All categorical variables were analysed using chi-square test while logistic regression was used for continuous independent variables and categorical outcome. Multivariate logistic regression was used for variables with p-value <0.05 using ENTER method. p-value less than 0.05 was considered statistically significant. Difference in difference test was used to analyse the difference between the proportions of VRE positive cases in MICU.
Results
In MICU, out of the 302 patients studied, 83 (27.4%) were colonized with VRE and 219 were negative. Among the 32 VRE negative patients from whom rectal swabs were periodically collected, 5 patients acquired the VRE colonization during the ICU stay. With this, the final VRE colonization rate in MICU increased from 27.4% to 29%. Of the total VRE isolates, 68(77.2%) were identified as E.faecium and 20 (23.6%) as E.faecalis. The MIC values of all the VRE strains for vancomycin and teicoplanin ranged from 64 to 256 mg/L and all were of vanA phenotype. Vancomycin resistance gene vanA was detected in all the VRE isolates from colonized patients. None of the isolates were positive for vanB, vanC1 or van C2/C3 genes by PCR [Table/Fig-2].
Detection of vancomycin resistant gene vanA by PCR (732 b.p).
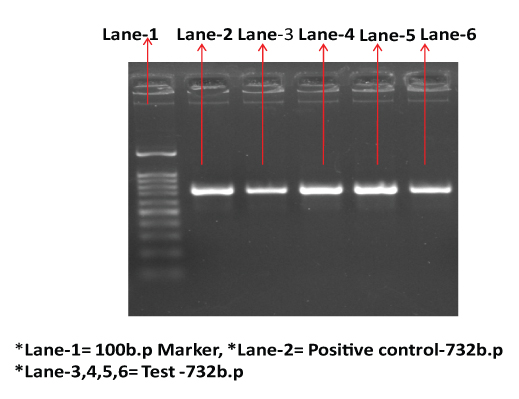
Among the patients admitted to MICU, the risk factors found to be significantly associated with VRE colonization were younger age (for one unit change in the age there will be 3 times less odds of developing VRE colonization), longer duration of hospital stay prior to collection of the specimen (8.20 days among colonized patients vs 4.50 days among those not colonized) as also consumption of vancomycin (p=0.048) and ceftriaxone (p=0.25) [Table/Fig-3,4].
Demographic features and risk factors associated with VRE colonization among patients admitted to MICU (n=302) (Logistic regression analysis).
| Variables | VREcolonisationn=83(%) | VRE notpresentN=219 (%) | OR(95% -CI) | p-value |
|---|
| Age in years[mean(SD)] | 37.9(13.9) | 41.6(13.4) | 0.979(0.96-0.99) | 0.030 |
| Female | 45(30.8) | 101(69.2) | 0.78(0.403-1.512) | 0.462 |
| Male | 38(24.4) | 118(75.6) | 1 |
| Urinarycatheterization | 69(83.1) | 192(87.7) | 1.49(0.726-3.076) | 0.276 |
| No urinarycatheterization | 14(16.9) | 27(12.3) | 1 |
| Duration of currentHospital stay beforespecimen collection[Mean(SD)] | 8.20(5.2) | 4.50(2.9) | 1.24(1.165-1.337) | 0.00 |
| H/O Previoushospitalization | 12(14.4) | 37(16.8) | 0.83(0.410-1.685) | 0.608 |
| No H/O Previoushospitalization | 71(86.4) | 182(84.2) | 1 |
| H/O Surgery | 9(10.8) | 24(11) | 1.49(0.7263.076-) | 0.276 |
| No H/O surgery | 74(89.2) | 195(89) | 1 |
| Diabetic | 14(23.7) | 45(76.3) | 0.78(0.403-1.512) | 0.462 |
| Non Diabetes | 69(28.5) | 174(71.5) | 1 |
| Renal failure | 9(10.8) | 19(8.7) | 1.28(0.554-2.956) | 0.563 |
| Non renal failure | 74(89.2) | 200(91.3) | 1 |
| Malignancy | 3(3.6) | 10(4.6) | 0.78(0.210-2.921) | 0.717 |
| Non malignancy | 80(96.4) | 209(95.4) | 1 |
| Vancomycin given | 49(35.3) | 90(64.7) | 2.07(1.23-3.45) | 0.006 |
| Vancomycinnot given | 34(20) | 129(79.1) | 1 |
| Ceftriaxonegiven | 64(32) | 136(68.0) | 2.05(1.15-3.67) | 0.015 |
| Ceftriaxonenot given | 19 (18.6) | 83(81.4) | 1 |
| Metronidazolegiven | 57(26.5) | 159(73.5) | 0.83(0.48-1.44) | 0.514. |
| Metronidazolenot given | 26(30.2) | 60(69.8) | 1 |
| Meropenemgiven | 37(28.7) | 92 (71.3) | 1.11(0.667-1.848) | 0.687 |
| Meropenemnot given | 46(26.6) | 127 (73.4) | 1 |
| Cefoperazonesulbactamgiven | 21(28.8) | 52(71.2) | 1.08(0.606-1.952) | 0.778 |
| Cefoperazonesulbactamnot given | 62(27.1) | 167(72.9) | 1 |
| Azithromycin given | 33(27.7) | 86(72.3) | 1.01.(604-1.698) | 0.961 |
| Azithromycin not given | 50(27.5) | 133(72.5) | 1 |
| Amikacin given | 51(29.7) | 121(70.3) | 1.29(0.770-2.163) | 0.332 |
| Amikacin not given | 32(24.6) | 98(75.4) | 1 |
| Levofloxacin given | 19(27.1) | 51(72.9) | 0.97(0.537-1.783) | 0.942 |
| Levofloxacin not given | 64(27.6) | 168(72.4) | 1 |
| Gentamycin given | 35(26.3) | 98(73.7) | 0.90 (0.540-1.500 | 0.687 |
| Gentamycin not given | 48(28.4) | 121(71.6) | 1 |
Risk factors associated with VRE colonization among patients admitted to MICU(n=302) {Multivariate (adjusted) Logistic regression analysis}.
| Variables | VREcolonisationn=83(%) | VRE notpresentN=219 (%) | OR(95% -CI) | p-value |
|---|
| Age in years[mean(SD)] | 37.9(13.9) | 41.6(13.4) | 0.97(0.97-0.95) | 0.035 |
| Vancomycin given | 49(35.3) | 129(79.1) | 1.78(1.0-3.15) | 0.048 |
| Vancomycin not given | 34(20) | 90(64.7) | 1 |
| Ceftriaxone given | 64(32) | 136(68.0) | 2.08(1.09-3.97) | 0.025 |
| Ceftriaxone not given | 19 (18.6) | 83(81.4) | 1 |
| Duration of currentHospital stay beforespecimen collection[Mean(SD)] | 8.20(5.2) | 4.50(2.9) | 1.24(1.16-1.33) | 0.001 |
During the study period, five patients from MICU were infected with VRE, 4 among them being previously colonized with VRE. On the other hand, none of the remaining 84 colonized patients developed infection with VRE during their entire hospital stay. The species and antimicrobial susceptibility profiles of the infecting and colonizing isolates were similar in all cases. The average duration between detection of colonization and infection was 6.5 days. The commonest infection was urinary tract infection followed surgical site infections.
All isolates of the VRE from MICU were resistant to teicoplanin. In addition, very high rate of resistance to other antibiotics was noted (88%, 82% and 84% to ampicillin, high level gentamycin and tetracycline respectively). No resistance was observed to linezolid [Table/Fig-5].
Resistance of VRE isolates to other antibiotics (n=83).
| Teicoplanin | Ampicillin | High level Gentamycin | Tetracycline | Linezolid |
|---|
| 83 (100%) | 73 (88 %) | 68 (82 %) | 70 (84 %) | 0 |
Discussion
Of all the 19 number of ICU’s catering to adult patients in JIPMER, MICU was chosen to represent a heterogeneous population of adults at risk for VRE colonization. The rate of VRE colonization among patients admitted in MICU in the present study was 29%. The rate of VRE colonization in published reports showed a distinct geographic variation with USA reporting higher rates (12.3%) when compared to Europe (2.7%), South America (7%) Asia (5.3%) and Oceania (4.4%) [11]. Although the average rate of VRE colonization in USA hospitals is 12.5% according to the meta analysis of Zakias et al., there were a few hospitals reporting much higher rates of 42% at admission which is even higher than the rates encountered in the present study [11]. The differences in the published rates of VRE colonization may reflect differences in the infection control practices, antibiotic consumption policies, cultural differences among health care personnel and the methodologies followed for detection of colonization [11].
In the meta-analysis by Ziakas et al., the average rate was reported to be 6.3-9% at initial admission while an additional 6.9-11% acquired VRE during their ICU stay [11]. We followed up 32 initially negative patients for subsequent colonization and found 5/32 (15.6%) of them getting colonized which is higher than that reported by Ziakas et al., [11].
The risk factors for VRE colonization were assessed based on the previous studies which reported haemodialysis, consumption of vancomycin, use of third generation cephalosporins, exposure to meropenem, increased hospital stay, chronic renal failure, patients with invasive devices, abdominal surgery, bedsores and MRSA co-colonization as being associated with increased chances of VRE colonization [5–7,12–14]. Our study illustrated that younger age group, increased length of hospital stay (8.20 days), consumption of vancomycin and ceftriaxone were significantly associated with VRE colonization in MICU patients. Longer duration of hospital stay prior to being colonized has been found an independent risk factor for VRE colonization. This has been postulated to be due to the higher probable antibiotic exposure in these patients combined with their proximity to an already colonized patient [15].
Although VRE colonization has been found to increase the risk for ensuing VRE infections, this finding is not universal with rates ranging from 0-45% being reported in various studies whereas the risk among non-colonized patients is less than 2% [11]. This wide range may be reflective of the patient population studied as those in general wards are at a lower risk as compared to those with solid organ transplants or hematological malignancies. These differences have also been attributed to variations in the virulence of the colonizing strains [11]. In the present study, 4 of the 88 colonized patients went on to develop VRE infections (4.5%) while only one of the 214 non colonized patients was infected. Based on the same species and similar antimicrobial susceptibility profiles between colonizing and infecting isolates in all the cases we came to the purely speculative conclusion that the same strain went on to cause infection. However molecular tests such as Multi Locus Sequence Typing (MLST) or Pulsed Field Gel Electrophoresis (PFGE) would have confirmed the genetic relatedness between the isolates. In one such study published by Papadimitriou-Olivgeries et al., found 4 out of 107 colonized patients developing infection with the same strain [15]. They made an important observation that VRE colonized patients may also develop subsequent infections with VSE isolates (proved by the same PFGE type).
In this study we looked for the presence of vanA, vanB and vanC genes out of several drug resistance marker genes designated for VRE as vanA & vanB genes are the most common ones followed by van C [1]. All the VRE strains isolated from colonized patients belonged to vanA phenotype and it was confirmed by vanA gene specific PCR. None of the isolates were positive for vanB, vanC1 & vanC2/C3 genes. There is a global variation between the different van phenotypes and genotypes encountered. In USA and Israel, vanA phenotype accounts for majority of the VRE, while in Australia, it is the vanB phenotype which predominates [16]. In our own hospital, a few years ago, we had reported both vanA and vanB phenotypes of VRE although in that study VRE isolates from across the hospital (from both ICU and non-ICU patients) were included [17].
Another potentially deleterious effect of increase in VRE colonization and infection is the risk of developing vancomycin resistance in Staphylococcus aureus strains [18]. Vancomycin resistance in S.aureus may be acquired by plasmid mediated transmission from glycopeptide resistant Enterococci strains [18].
Conclusion
Preventive measures need to be taken especially in ICU’s to curtail the spread of vancomycin resistance among Enterococci. Although isolation or cohorting of colonized patients may be ideal, they may not be very practical. Instead strict adherence to hand hygiene and education of health care workers may be more achievable methods of infection control.