Efficacy and Safety of Complete RAAS Blockade with ALISKIREN in Patients with Refractory Proteinuria Who were already on Combined ACE Inhibitor, ARB, and Aldosterone Antagonist
Prabitha Panattil1, M Sreelatha2
1 Assistant Professor, Department of Pharmacology, Government Medical College, Kottayam, Kerela, India.
2 Professor and Head of Department, Department of Nephrology, Government Medical College, Calicut, Kerela, India.
NAME, ADDRESS, E-MAIL ID OF THE CORRESPONDING AUTHOR: Dr. Prabitha Panattil, Assistant Professor, Department of Pharmacology, Government Medical College, Kottayam-686008, Kerela, India.
E-mail: prabithasreejith@gmail.com
Introduction
Proteinuria is always associated with intrinsic kidney disese and is a strong predictor of later development of End Stage Renal Disease (ESRD). As Renin Angiotensin Aldosterone System (RAAS) has a role in mediating proteinuria, inhibitors of this system are renoprotective and patients with refractory proteinuria are put on a combination of these agents. The routinely employed triple blockade of RAAS with Angiotensin Converting Enzyme (ACE) inhibitor, ARB and Aldosterone antagonist has many limitations. Addition of Aliskiren to this combination suppresses the RAAS at the earliest stage and can offset many of these limitations.
Aim
This study was conducted to assess the safety and efficacy of complete RAAS blockade by the addition of Aliskiren in those patients with refractory proteinuria who were already on triple blockade with ACE inhibitor, ARB and Aldosterone antagonist.
Settings
This study was conducted in Nephrology Department, Calicut Medical College.
Materials and Methods
A total of 36 patients with refractory proteinuria who were already on ACE inhibitor, ARB and Aldosterone antagonist were divided in to two groups A and B. Group A received Aliskiren in addition to the above combination whereas group B continued the same treatment for 12 weeks. Efficacy of the treatment was assessed by recording 24hr urine protein and safety by S.Creatinine, S.Potassium every 2 weeks of the treatment period.
Statistical Analysis
Statistical analysis of the lab values was done using SPSS software. Unpaired t-test, Paired t-test and Chi-square test were done for data analysis.
Results
Statistical analysis revealed that addition of Aliskiren to the combination therapy with ACE inhibitor+ ARB+ Aldosterone antagonist offers no advantage. But mean reduction in proteinuria was more with Group A than Group B. There is no statistically significant change in S.Creatinine and S.Potassium at the end of treatment.
Conclusion
As proteinuria is a strong risk factor for progression to ESRD, even a mild decrease in proteinuria by treatment is renoprotective. Hence treatment with group A may be considered clinically superior to group B with no alteration in safety and tolerability. But further multicentre studies with larger sample size and dose escalation are required for confirmation.
Direct renin inhibitor, End stage renal disease, Nephrotic syndrome
Introduction
Proteinuria is increased rate of excretion of protein in urine. Proteinuria >150mg/24hr is abnormal. It is almost always associated with intrinsic kidney disease and strong predictor of later development of End Stage Renal Disease (ESRD) [1,2]. Whenever proteinuria is decreased by treatment progression to ESRD is reduced. Clinical trials have consistently shown that antiproteinuric treatment maximise renal protection. There are three different types of proteinuria. They are glomerular, tubular and overflow proteinuria [3]. Among these the most clinically important one is glomerular proteinuria.. Nephrotic syndrome is a type of glomerular proteinuria and is characterised by proteinuria >3.5g/24hr. Conventional treatment of nephrotic syndrome is with corticosteroids and immunosuppressants [4]. But there are cases resistant to the conventional treatment and is termed as refractory proteinuria. Stimulation of Renin Angiotensin Aldosterone System (RAAS) has a role in genesis of glomerular lesions leading to proteinuria [5]. Inhibition of RAAS is one of the most powerful maneuvers to slow progression of renal disease and is called as renoprotective agents. The drugs that block RAAS are Angiotensin converting enzyme inhibitors (ACE inhibitors), AT1 Receptor Blockers (ARB), Aldosterone antagonist and Direct Renin Inhibitors (DRI). Patients diagnosed to have refractory proteinuria are put on a combination of ACE inhibitors, ARB and Aldosterone antagonist [6]. But this combination has many limitations [7]. Addition of DRI to this combination suppresses the RAAS at the earliest stage and can offset many of these limitations. As it inhibits the rate limiting step in RAAS cascade, synthesis of all subsequent components of the cascade are reduced and thus DRI causes more complete blockade and mitigates proteinuria [8]. Aliskiren is a DRI. This study was conducted to assess the safety and efficacy of complete RAAS blockade by the addition of Aliskiren in those patients with refractory proteinuria who are already on triple blockade of RAAS with ACE inhibitor, ARB and Aldosterone antagonist. This study also aims to assess the superiority of addition of Aliskiren to the triple blockade of RAAS in refractory proteinuria.
Materials and Methods
This study designed as prospective observational study was approved by Institutional ethics committee. Sample size was calculated according to the study – “Triple blockade of RAAS in non-diabetic chronic kidney disease an open label cross over randomized controlled trial” [9]–and was found to be 15 (15 patients in each group). A total of 36 patients diagnosed to have refractory glomerular proteinuria (Resistant to conventional immunosuppressive treatment) in the age group of 5-50years, who were on triple combination therapy with ACE inhibitor, ARB and Aldosterone antagonist with GFR>60ml/mt were enrolled in the study from two different units of Nephrology Dept, Calicut Medical College. The study period was from August 2009 to August 2010. Exclusion criteria were pregnancy, lactation, GFR<60ml/mt and diabetes mellitus. GFR is approximately equal to creatinine clearance and was calculated by applying Cockroft -Gault formula.
According to Nephrology Unit 1 protocol, aliskiren was added to the above mentioned triple combination in patients with refractory proteinuria whereas in Nephrology Unit 2 the triple combination therapy was continued as such. Among the 36 recruited patients, 21 patients received treatment from Nephrology unit 1, and 15 patients from Unit 2. Nephrology unit 1 treatment named as Group A was the combination of following drugs.
| ACE inhibitor | Ramipril 5mg od/ Enalapril 10mg od |
| ARB | Telmisartan 80mg od/ Losartan 100mg od |
| Aldosterone antagonist | Spironolactone 25mg od |
| Direct Renin inhibitor | Aliskiren 150mg daily |
The other patients recruited from Nephrology Unit 2 were to continue the triple combination (ACE inhibitor + ARB+ Aldosterone antagonist) and named as Group B [Table/Fig-1].
Flow diagram depicting the prospective observational study of efficacy of aliskiren in those patients with refractory proteinuria who are already on combined ACE inhibitor, ARB and Aldosterone antagonist.
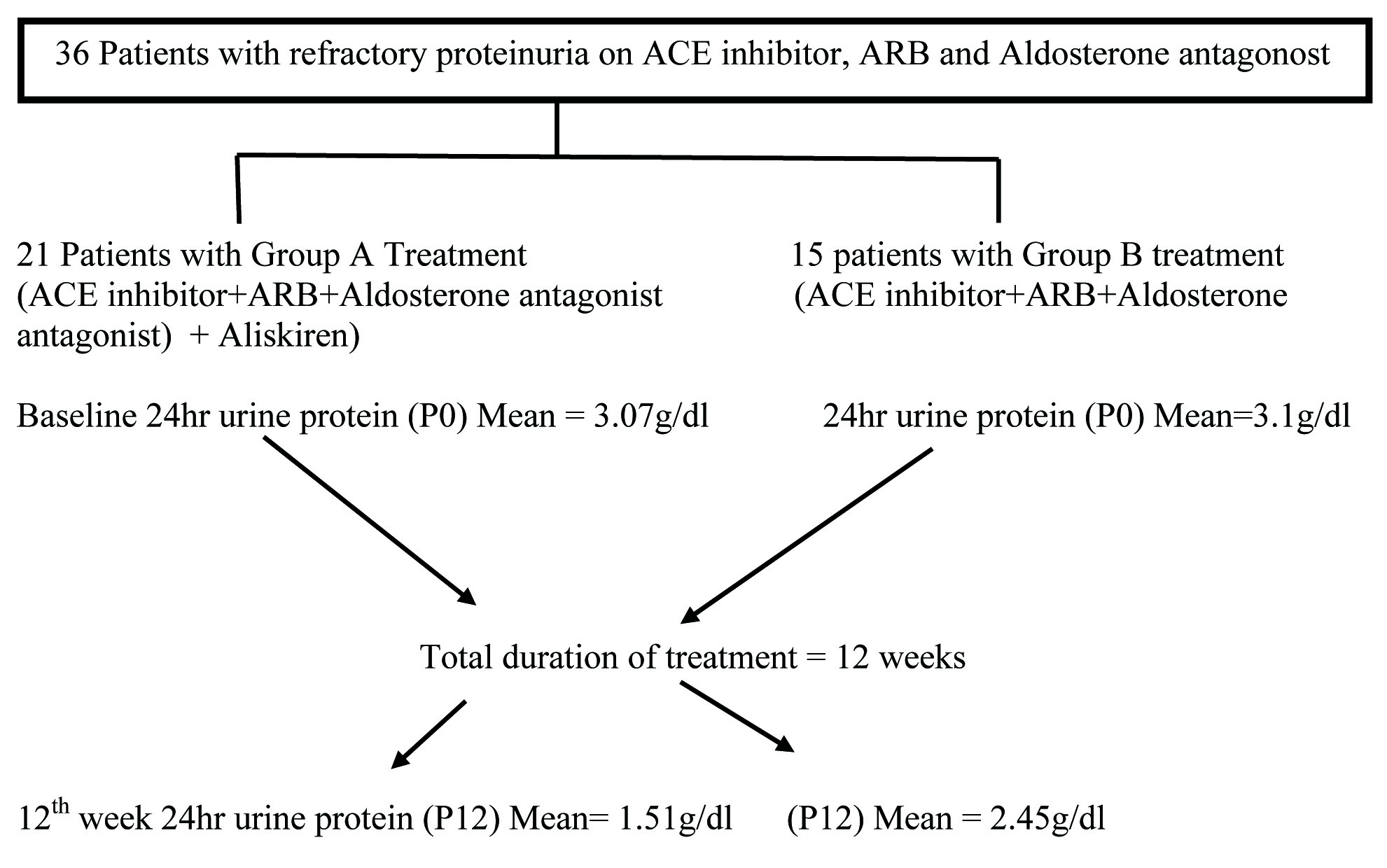
All drugs except aliskiren were provided from the medical college pharmacy. Patients were asked to buy Aliskiren from outside as it was not available in medical college pharmacy. Total duration of treatment was 12 weeks. Informed consent was taken from all recruited patients. Complete history was elicited which covered personal data, duration of illness and other modalities of treatment as mentioned in proforma. Diagnosis was noted down according to the renal biopsy report. Thorough physical examination was done in two groups. Lab tests such as baseline 24hr urine protein (P0), S.Creatinine (C0), and Potassium (K0) were done. Review was done on 2nd, 4th, 8th, 10th and 12th week of treatment period. The above mentioned lab tests were repeated during each visit. Safety was assessed by recording all adverse events and patients were instructed to report immediately if any untoward effects occurred. 3 among 21 patients who received treatment with Group A did not complete treatment. Two of them were drop outs during 8th and 10th weeks of treatment period. One patient reported 3weeks after starting treatment with increased oedema and hyperkalaemia following which the treatment was stopped. As per Naranjo algorithm the causality relationship of this adverse reaction was assessed as possible. Patients not responding to 12 weeks treatment were continued with non-specific reno protective regimen. (ACE inhibitor /ARB/Aldosterone antagonist).
Statistical Analysis
Results were analysed using SPSS software. Unpaired t-test, Paired t-test and Chi-square test were done for analysis of data. Results were tabulated and significance was expressed according to the p-value<0.05(significant) and < 0.001 (Highly significant).
Results
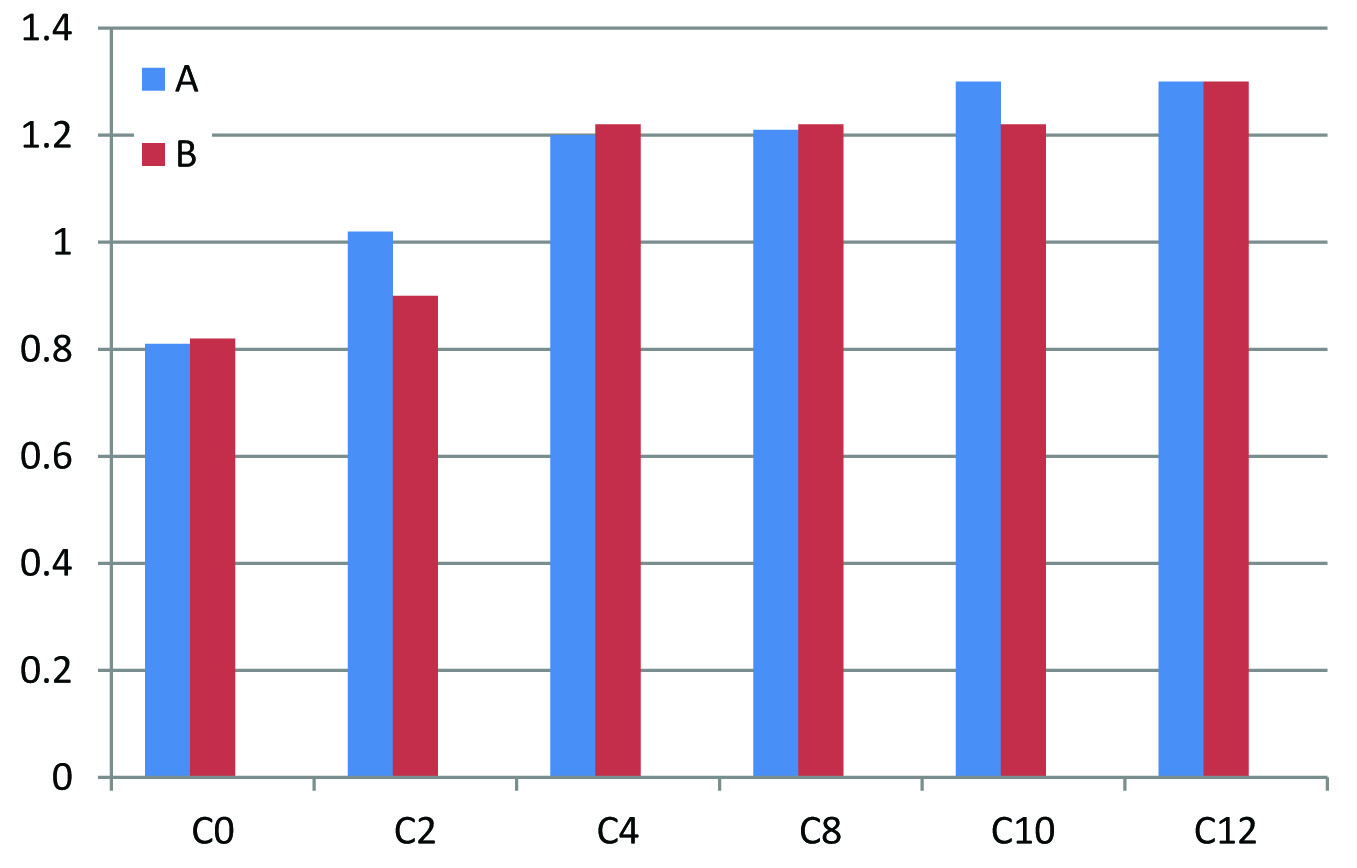
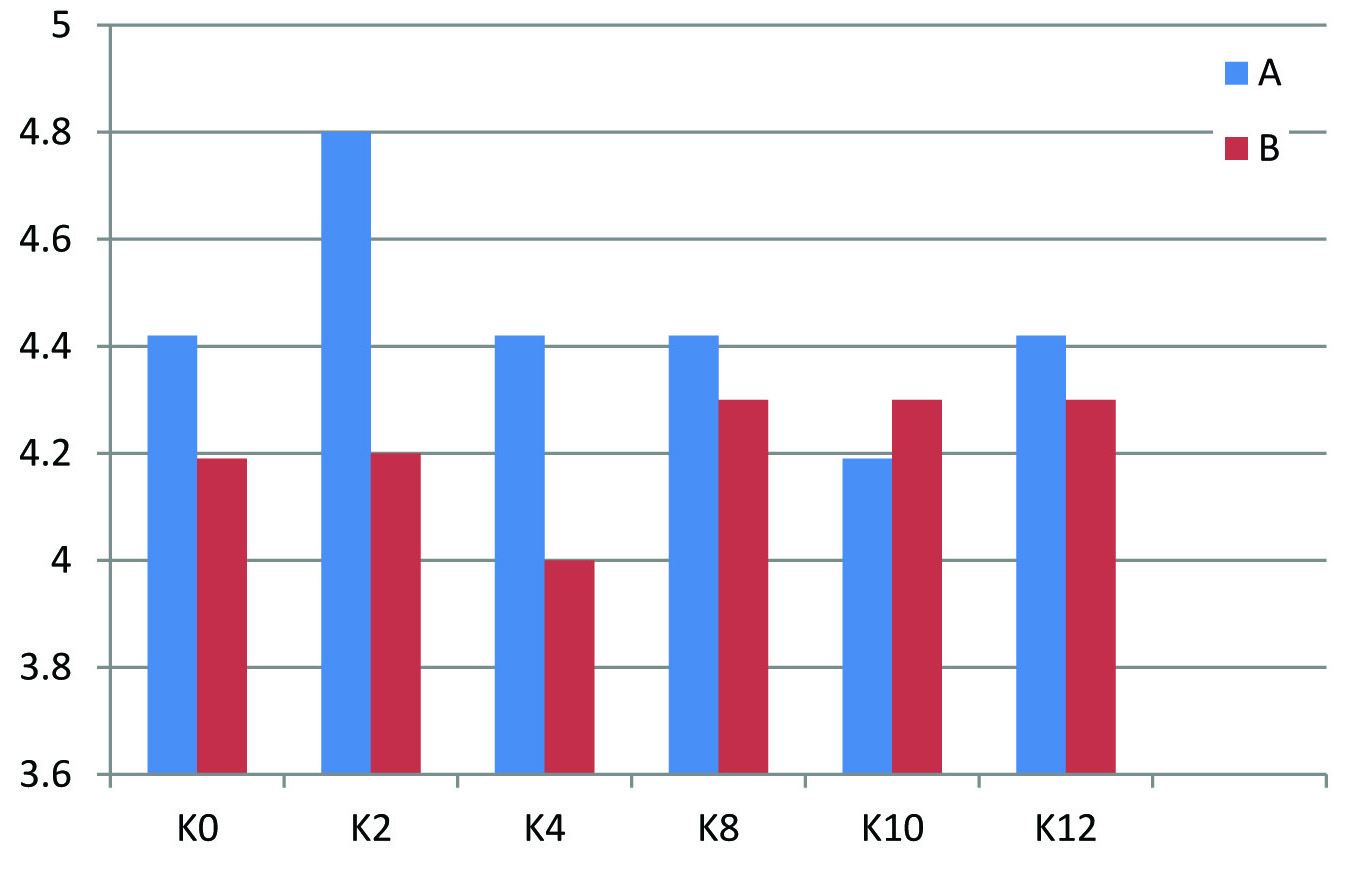
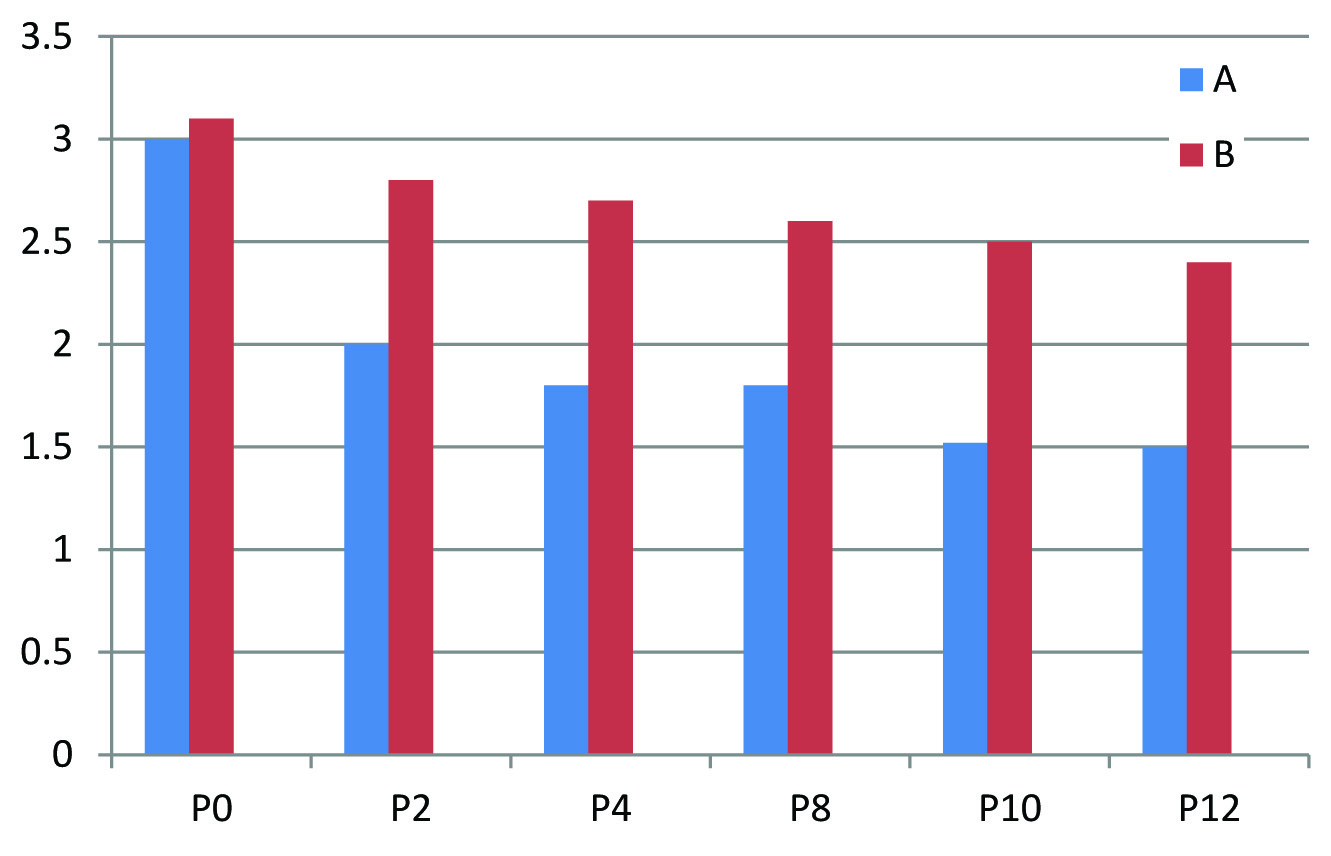
Baseline parameters such as age, sex, place and laboratory values of both groups A and B were assured to be comparable. Paired t-test was used to assess the difference in proteinuria before starting the treatment and after the completion of treatment. p-value for Group A is 0.009 (<0.05) and is significant whereas that of Group B is 0.174 (>0.05) and is not significant. Mean reduction in proteinuria with Group A is 1.56g with that of Group B is 0.74g. But when unpaired t-test was employed superiority of Group A over Group B was not statistically significant (p-value at 12th week =0.278>0.05) [Table/Fig-2]. There is no statistically significant difference in S.potassium, S.creatinine values at the end of treatment when both groups are compared indicating that Group A and Group B has equal safety and tolerability profile [Table/Fig-3,4].
Showing comparison of changes in lab values before (0 week) and after treatment (12th week) between the two groups.
| Changes in lab values before and after treatment | Group | #N | Mean | SD | p-value |
|---|
| *P0-P12 | A | 18 | 1.56 | 2.25 | 0.278 |
| B | 15 | 0.74 | 1.99 |
| **K0-K12 | A | 18 | 0.03 | 0.74 | 0.446 |
| B | 15 | 0.15 | 0.56 |
| ***C0-C12 | A | 18 | 0.47 | 0.49 | 0.914 |
| B | 15 | 0.45 | 0.45 |
# N- number of patients enrolled for study
*Reduction in proteinuria at 12th week
**Change in S. Potassium at 12th week
***Change in S.Creatinine at 12th week
Changes in S.Creatinine with Group A and Group B.
X axis – C0 - S.Creatinine before treatment (0 week), C2 – S.Creatinine on 2nd week, C4 – S. Creatinine on 4th week, C8 – S.Creatinine on 8th week, C10 – S.Creatinine on 10th week, C12 – S.Creatinine on 12th week
Y axis – S.Creatinine in mg/dl
Changes in S.Potassium with Group A and Group B.
X axis – K0- S.Potassium at before treatment (0 week), K2 – S. Potassium on 2nd week, K4- S.Potassium on 4th week, K8 – S.Potassium on 8th week, K10 – S.Potasssium on 10th week, K12- S.Potassium on 12th week
Y axis – S.Potassim values in mEq/L
Discussion
Proteinuria is a strong risk factor for progression of chronic kidney disease and its reduction improves renal function to a great extent. Hence antiproteinuric treatment offers immense renal protection. Refractory proteinuria is important because it is linked to the development of ESRD. The drugs that block RAAS such as ACE inhibitors, ARB and Aldosterone antagonist are routinely employed as renoprotective agents in refractory proteinuria. But these agents when used as mono therapy/ dual combination therapy [10] cannot cause complete blockade of RAAS and have their own limitations and side effects on its prolonged use. It is better to administer these three drugs together which cause triple blockade of RAAS and offset the problems met with their individual use and hence this triple blockade of RAAS is routinely used in refractory proteinuria. But due to remarkable complexity of RAAS, the triple blockade is not adequate to suppress its activity. The main limitations are [7,8]:
ACE inhibitor and ARB, interrupt both short loop and long loop negative feedback mechanisms and result in high Plasma Rennin activity (PRA) which may overcome the drugs’ effectiveness.
Renin directly stimulates mitogen activated protein kinase and also it has direct profibrotic role.
ARBs leave the AT2 receptor unblocked and these AT2 receptors are involved in renal inflammation by activation of NF-k B and induction of chemokines.
Taking all these mechanisms together DRI offers a more complete inhibition of RAAS. Aliskiren which is a DRI inhibit the rate limiting step in RAAS cascade and block the synthesis of all subsequent components of the cascade. This study assessed the efficacy and safety of complete RAAS blockade with DRI in those patients with refractory proteinuria who are already on ACE inhibitor, ARB and aldosterone antagonist. After statistical analysis it was demonstrated that addition of aliskiren to the combination therapy with ACE inhibitor, ARB and Aldosterone antagonist offers no advantage. But even though there was no statistical significance, mean reduction in proteinuria was more with Aliskiren group (Group A) [Table/Fig-5]. A 24hr urine protein values correlates significantly with GFR decline and progression of chronic renal disease to ESRD [11], whenever proteinuria is decreased by treatment progression, ESRD is reduced. Hence even a slight decrease in urine protein excretion improves renal function to a great extent. Thus Aliskiren+ACE inhibitor+ARB+Aldosterone antagonist combination is clinically superior to ACE inhibitor+ARB+ Aldosterone antagonist combination, with no alteration in safety and tolerability. Comparing with other studies, this study resulted in a moderate reduction (> 50%) in proteinuria [12].
Mean reduction in proteinuria with Group A and Group B.
X axis – P0 – Proteinuria before treatment (0 week), P2 – Proteinuria on 2nd week, P4 – Proteinuria on 4th week, P8 – Proteinuria on 8th week, P10- Proteinuria on 10th week, P12 – Proteinuria on 12th week
Y axis – 24hr urine protein values in grams
Limitation
The reduction in proteinuria was more with Group A compared to Group B. But this difference in 24hr urine protein was not statistically significant. Multicentre studies, bigger sample size and extended duration of treatment are required to reject null hypothesis. Fixed dose of aliskiren was given during the entire treatment period. Dose escalation was not done during the treatment period for better outcome as done in other studies. This is the first study of its kind in which aliskiren was added to the combination of ACE inhibitor + ARB+ Aldosterone antagonist. Based on this study, further studies should be conducted with more patients.
Conclusion
According to this study, addition of Aliskiren to combination therapy with ACE inhibitor+ARB+Aldosterone antagonist is clinically more efficacious than triple combination therapy with ACE inhibitor+ARB+Aldosterone antagonist in refractory proteinuria and the two modes of treatment has almost equal safety profile.
# N- number of patients enrolled for study
*Reduction in proteinuria at 12th week
**Change in S. Potassium at 12th week
***Change in S.Creatinine at 12th week
[1]. Proteinuria. National institute of diabetes and kidney diseases. Updated Dec 18 2013. Available from www.niddk.nih.gov/health-information//health topics/kidney/proteinuria [Google Scholar]
[2]. Sundin PO, Udumyan R, Sjostorm P, Predicators in adolescene of ESRD in middle aged menAm J kidney Dis 2014 64(5):723-29. [Google Scholar]
[3]. Lerma EV. Proteinuria. Available online emedicine.medscape.com. Last updated on Dec 2015 [Google Scholar]
[4]. Lewis JB, Nelson EG, Glomerular diseases. Diseases of kidney and urinary tract inHarrison’s Principle of Internal Medicine 2 (18):2319 [Google Scholar]
[5]. Bargman JM, Korecki KS, Chronic kidney disease. Diseases of kidney and urinary tract InHarrison’s Principle of Internal Medicine2(18):2319 [Google Scholar]
[6]. Remission clinic task forceClinical research. Aldocele Daco. The remission of clinic approach to halt the progression of kidney diseaseJ Nephrol 2011 24(3):274-81. [Google Scholar]
[7]. Alfie Aparico LS, Waisman GD, Current strategies to achieve further cardiac and renal protection through enhanced renin angiotensin aldosterone inhibitionRev Recent Clinical Trials 2011 6(2):134-46. [Google Scholar]
[8]. Simeoni M, Nicotera R, Colao M, Direct inhibition of plasmatic rennin activity with aliskiren a promising but under investigated therapeutic option for non-diabetic glomerulonephritisInt Urol Nephrol 2016 48(2):229-37. [Google Scholar]
[9]. Tylicki L, Rut Kowsi, Renke M, Triple blockade of RAAS in nondiabetic chronic kidney disease;-an open label cross over randomized controlled trialAmerican Medical Journal of Kidney Diseases 2008 52(3):486-93. [Google Scholar]
[10]. Bolignano D, Pulmerse Navaneethan SD, Aldosterone antagonists for preventing a progression of CKDCochrane data base Syst Rev 2014.April 29 4:CD007004doi 16-1002/14651858 CD007004 pub 3 [Google Scholar]
[11]. Teo BW, Loh PT, Wong WK, Spot urine estimations are equivalent to 24hr urine assessments of urine protein excretion for predicting clinical outcomesInternational Journal of Nephrology 2015 2015:15684 [Google Scholar]
[12]. Lizakowski S, Tylicki L, Rutkowski B, Direct rennin inhibition –a promising strategy for renal protection?Med Sci Monit 2013 19:451-57. [Google Scholar]